Kyowa Hakko Kirin's Parkinson's therapy, Nourianz (istradefylline), gains FDA approval as an add-on to levodopa/carbidopa.

Kyowa Hakko Kirin's Parkinson's therapy, Nourianz (istradefylline), gains FDA approval as an add-on to levodopa/carbidopa.

Radicut/Radicava (edaravone), shown to slow the progression of ALS, is now approved for patients in China.

Orkambi and Symkevi have been rejected by the Scotland Medicines Consortium (SMC) due to their conclusion that the costs outweigh the benefits.

Vitrakvi (larotrectinib) recommended by the CHMP for approval in the EU.

 After 40 years with primary biliary cholangitis, Fiona's mother is still alive thanks to her daughter's dedication and a liver transplant.
After 40 years with primary biliary cholangitis, Fiona's mother is still alive thanks to her daughter's dedication and a liver transplant.
After 3 years of living with ALS, Marc found a new medicine on the other side of the world.
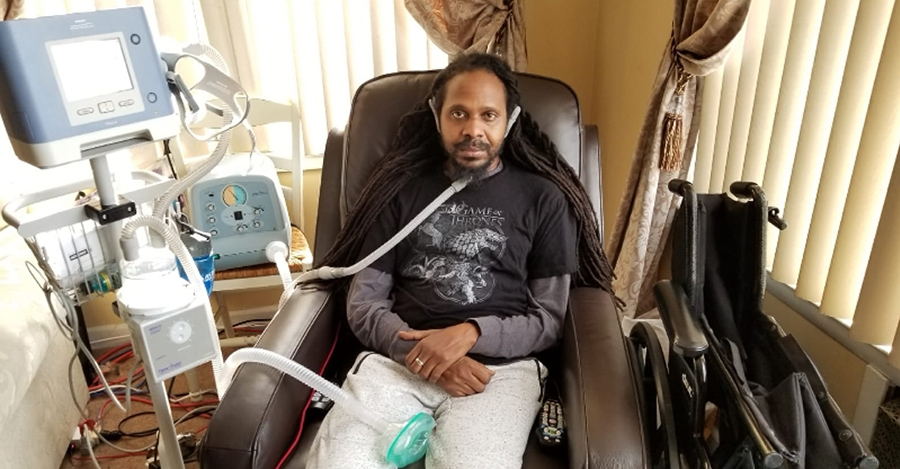
With tears, laughter and hope, Mark and his family find their own way to cope with ALS.

Diagnosed with ALS only 6 months ago, Peter went against the current to find more options to treat his disease.

MN-166 (ibudilast) will be part of the Phase III clinical trial on progressive multiple sclerosis subjects with secondary progressive MS without relapses.

New skin cancer medicine now approved in the EU for adults with metastatic or locally advanced cutaneous squamous cell carcinoma.

Still walking and talking after 4 years with ALS: we share Bakr's experience of living with a rare disease in the UAE to further his goal of creating awareness and to connect with other ALS patients.

Phase 2 clinical trial shows that ibudilast is linked to a 48% slowing in the progression of brain atrophy compared to placebo for patients with progressive MS.
Vyndaqel (tafamidis) is now approved in the U.S. after study showed increased survivorship rate and reduced hospital time due to heart related problems.

Approval for next phase of clinical trials puts ALS patients one step closer to accessing new treatment shown to slow disease progression.
Downloadable fact sheet answering the most common question TheSocialMedwork asked by healthcare providers.

Based on promising results from phase III clinical trials, Health Canada has decided to extend the use of Kalydeco (ivacaftor) to include children aged 1 to 2 years.

In recognition of Rare Disease Day, our colleague, Sera, shares her story of her dad's fight with Multiple System Atrophy (MSA).
A recent clinical trial indicates that Symkevi/Symdeko (tezacaftor/ivacaftor) can safely and effectively loosen thick, sticky mucus for children 6-11 years old.

The new site-agnostic cancer medicine, Vitrakvi (larotrectinib), gets accelerated approval from the U.S. Food and Drug Administration (FDA).

CHMP's assessment supports EMA's approval of non-small cell lung cancer (NSCLC) medicine, Vizimpro (dacomitinib).

Phase III trial shows that Copiktra (duvelisib) may be an option for patients with relapsed or refractory chronic lymphocytic leukaemia and small lymphocytic lymphoma.

Teresa's husband was diagnosed with ALS. She spent 11 years as his caregiver and discusses her experiences.

Onpattro (patisiran) is a first-in-class treatment that slows down the progression of hereditary transthyretin amyloidosis (hATTR).

After failing to show a clear benefit over chemotherapy on its own, both the FDA and EMA do not recommend Lartruvo (olaratumab) for use in combination with chemotherapy for new STS patients.

Realistic new year's resolutions for lasting results!

Over 50 countries have approved Spinraza (nusinersen), but not all have made it available to the patients who need it.

New medicine shows statistically significant improvements for prostate cancer patients.

We are looking for an outstanding Web Developer to be responsible for the coding, innovative design and layout of our website. 
A new biosimilar, Truxima (rituximab), shows comparable results to the reference medicine, Rituxan (rituximab), and takes FDA one step further toward expanding patient access to medicines.
