Learn about the new treatments for Parkinson's and how to get them in Chile before they're locally approved.


Learn about the new treatments for Parkinson's and how to get them in Chile before they're locally approved.


How can you access the latest treatments for lung cancer, before they're available in Poland?

The first medicine for fatty liver disease with fibrosis is not yet approved in Poland, but you can get it. Here's how.

What are the newest immunotherapies for cervical cancer, and how to get them in Poland right away?


When will the newest Alzheimer's medicine be available to patients in Switzerland (and how to get it sooner)?


Is the new Alzheimer's medicine available to Swiss patients, and how can you get it?


A new efficient and safe medicine to lower triglycerides in FCS patients is now available. But when will it be available to you?

When will the first approved medicine for fatty liver with fibrosis be available in Europe and the UK?


When will the new medicine for COPD be available to patients in Europe and the UK (and how not to wait)?

When will the first targeted therapy for CLDN 18.2-positive gastric and GEJ cancer be available to patients in Europe?
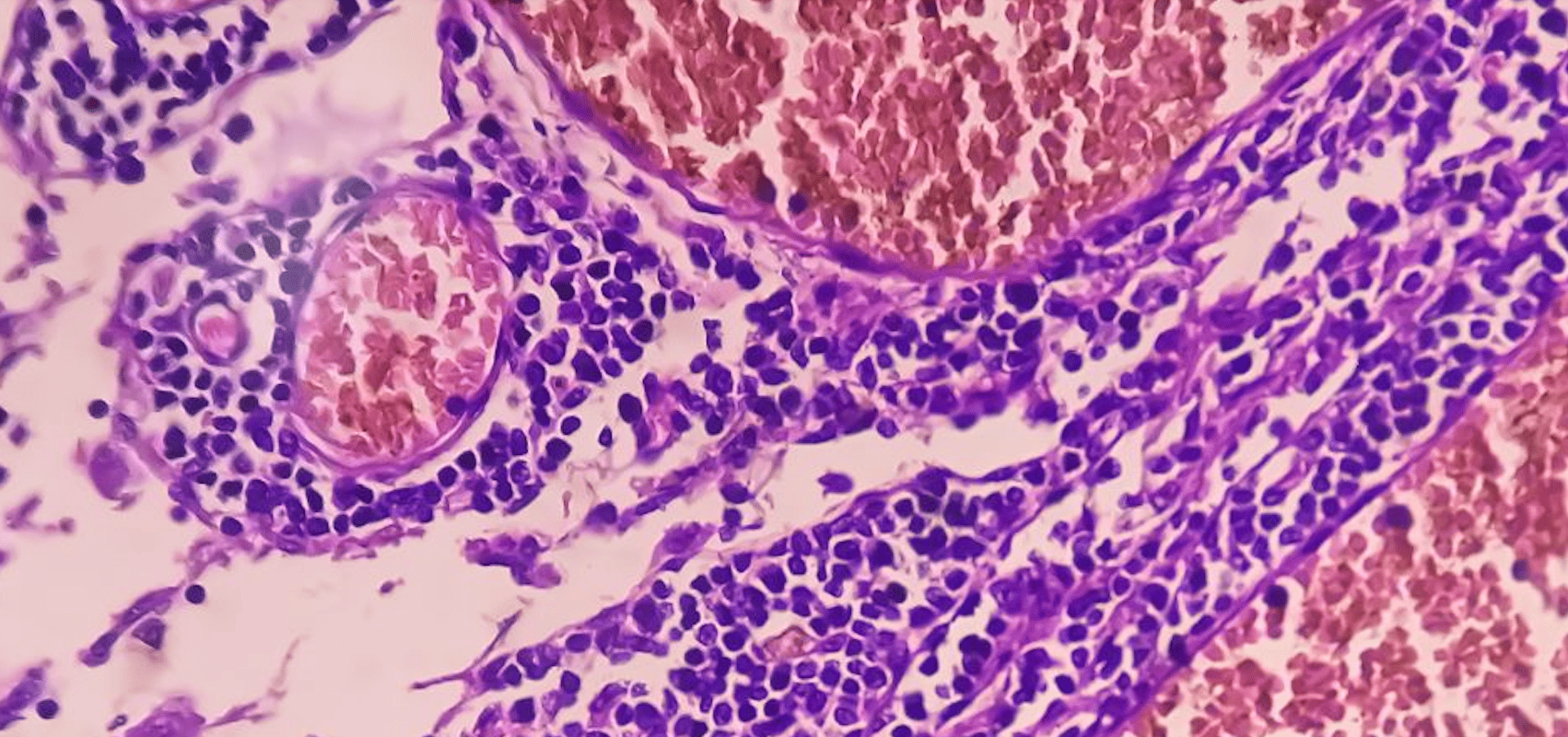

When will the first targeted therapy for acute leukemia with KMT2A translocation be available outside the USA?
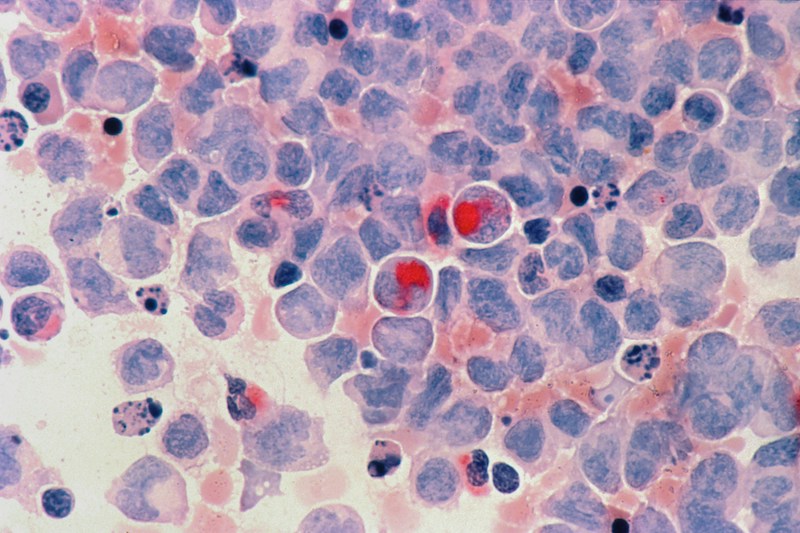

When will the first medicine for NPC be available to patients in Europe, the UK, and beyond (and how not to wait)?


When is the first targeted therapy for HER2-expressing BTC getting its approval? All about the timelines and your options in the meantime.


All the safe and legal options to access Leqembi while it's waiting for its EU approval.

Here is an overview of each medicine to help guide you and your physician when making a treatment decision for Cystic Fibrosis: 
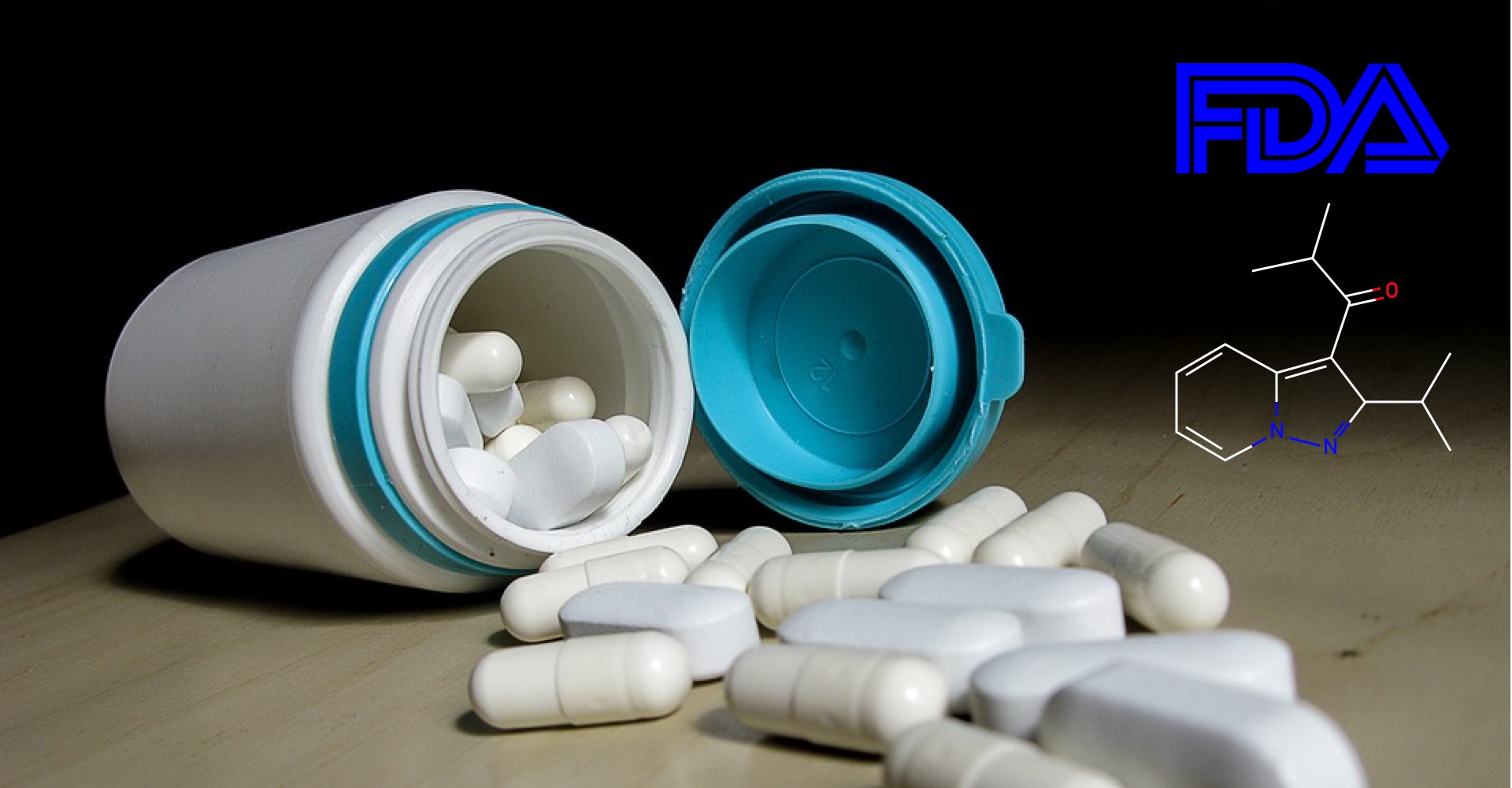
Kyowa Hakko Kirin's Parkinson's therapy, Nourianz (istradefylline), gains FDA approval as an add-on to levodopa/carbidopa.

Radicut/Radicava (edaravone), shown to slow the progression of ALS, is now approved for patients in China.

Orkambi and Symkevi have been rejected by the Scotland Medicines Consortium (SMC) due to their conclusion that the costs outweigh the benefits.

Vitrakvi (larotrectinib) recommended by the CHMP for approval in the EU.

New skin cancer medicine now approved in the EU for adults with metastatic or locally advanced cutaneous squamous cell carcinoma.

Vyndaqel (tafamidis) is now approved in the U.S. after study showed increased survivorship rate and reduced hospital time due to heart related problems.

Approval for next phase of clinical trials puts ALS patients one step closer to accessing new treatment shown to slow disease progression.
Based on promising results from phase III clinical trials, Health Canada has decided to extend the use of Kalydeco (ivacaftor) to include children aged 1 to 2 years.

A recent clinical trial indicates that Symkevi/Symdeko (tezacaftor/ivacaftor) can safely and effectively loosen thick, sticky mucus for children 6-11 years old.

Phase III trial shows that Copiktra (duvelisib) may be an option for patients with relapsed or refractory chronic lymphocytic leukaemia and small lymphocytic lymphoma.

Onpattro (patisiran) is a first-in-class treatment that slows down the progression of hereditary transthyretin amyloidosis (hATTR).

New medicine shows statistically significant improvements for prostate cancer patients.

Australia provides reimbursement for cystic fibrosis medicine.

The European Commission has approved Alunbrig (brigatinib) for the treatment of a specific type of lung cancer.
