What is Aimovig? | Information & Faq
Last updated: 14 October 2022
You can legally access new medicines, even if they are not approved in your country.
Learn howIf you suffer from migraine and you feel as if it’s impacting your relationships, work and personal life, you’re not alone. Migraine is the third most common disease worldwide, affecting around 1 in 7 people.
If you feel like you’ve used all treatment options and nothing has worked, you’re not alone either. Many migraine patients feel like painkillers and other migraine medicines do not work for them, or they can’t take them long-term.
What is Aimovig?
Aimovig (erenumab) is a preventive migraine treatment that works by blocking the activity of calcitonin gene-related peptide, a molecule that is involved in migraine attacks 1.
Who is Aimovig for?
Aimovig is indicated for use as a once monthly preventive treatment of adults who suffer from episodic and chronic migraine attacks2.
How is Aimovig taken?
The standard Aimovig instruction for dosage is:
- 70 mg injected subcutaneously (under the skin) once monthly
- Some patients may benefit from a dosage of 140 mg injected subcutaneously once monthly, which is administered as two consecutive subcutaneous Aimovig injections of 70 mg each
Aimovig is intended for patient self-administration.
Complete information about Aimovig dosing and administration can be found in the official prescribing information listed in our resources section.
Note: Please consult with your treating doctor for personalised dosing.
Are there any known side effects for Aimovig?
According to the official prescribing information, the most common side effects of Aimovig occurring with a frequency of at least 3% listed in the prescribing information include2:
- Injection site reactions
- Constipation
For a comprehensive list of side effects and adverse reactions please refer to the official prescribing information.
What are other available migraine treatments?
Emgality vs Aimovig and Ajovy vs Aimovig
Aimovig targets the CGRP receptor, whileAjovy & Emgality target the CGRP ligand to prevent migraine. The main difference is in dosing and administration. Aimovig is given as either a 70 or 140 mg monthly subcutaneous injection and comes as an auto-injector. Ajovy is dosed as 225 mg monthly or 675 mg quarterly subcutaneous injection and comes as a prefilled syringe with a small needle. Emgality is given as a loading dose of 2 injections of 120 mg each then a monthly 120 mg dose. Like Aimovig, Emgality comes as an auto-injector. Aimovig and Ajovy can be injected in the thigh, abdomen, or upper arm. Emgality has an additional injection site of the buttocks. All three are designed to be self-injected at home or by a healthcare professional during a visit to the clinic. The needles should be disposed of in a Sharps Container 6.
An alternative treatment is Vyepti, a CGRP receptor blocker, which is given 4 times per year (every 3 months) as intravenous (IV) treatment by a trained healthcare professional in a clinical setting7.
How effective is erenumab (Aimovig) according to the studies?
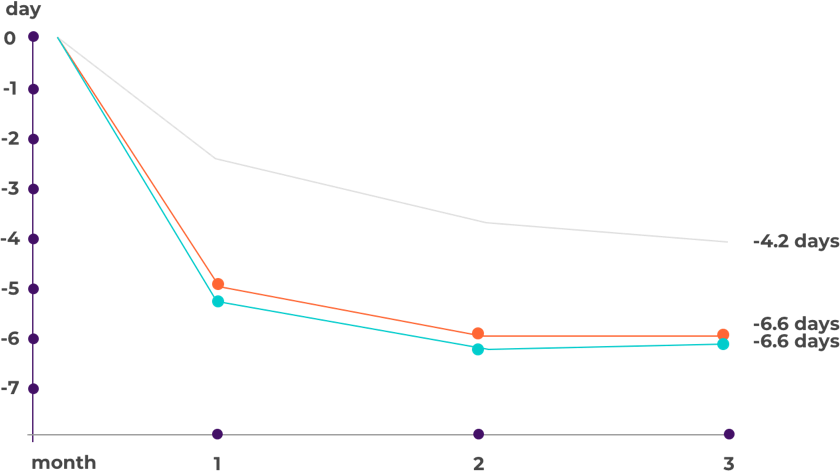
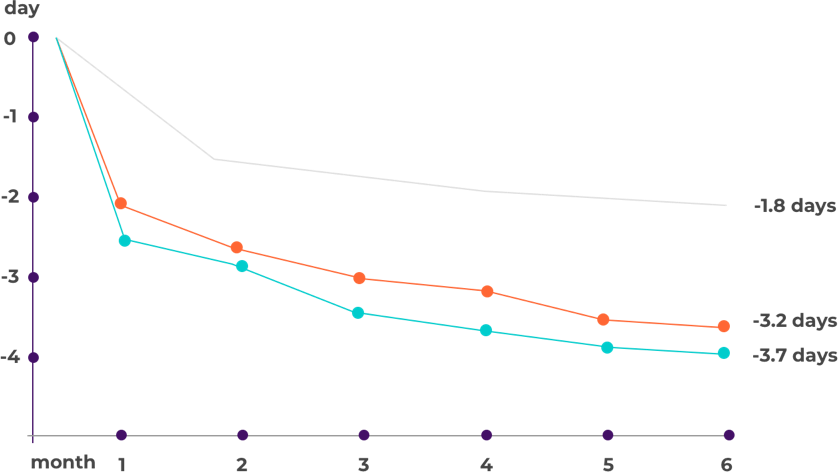
- Placebo
- Aimovig 70 mg
- Aimovig 140 mg
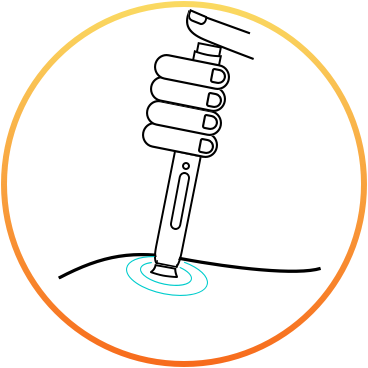
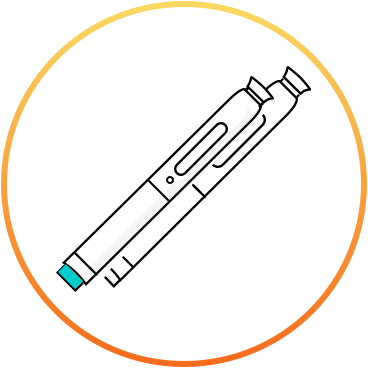
How to use the new migraine injection (erenumab)*?
How do these new blockers like erenumab (Aimovig) work?
Where has Aimovig been approved?
Aimovig was approved for the treatment of patients with migraine by:
- Food and Drug Administration (FDA), USA, on May 17, 2018
- Therapeutic Goods Administration (TGA), Australia, July 2, 2018
- Swissmedic, Switzerland, July 13, 2018
- European Medical Agency (EMA), European Union, July 26, 2018, indicated for the treatment in adults with 4 or more migraine days per month
- Health Canada, Canada, August 1, 2018, indicated for the treatment in adults with 4 or more migraine days per month
- Medsafe, New Zealand, January 16, 2020.
Please note that Aimovig approval may have also been granted in other regions than the ones we’ve listed.
If Aimovig is not available in your country, you might be able to purchase it from everyone.org. You can do so by making an Enquiry from our Aimovig medicine page.
References
1. FDA News Release: FDA approves novel preventive treatment for migraine, www.fda.gov, May 2018.
2. Summary of Product Characteristics [FDA]: AIMOVIGTM (erenumab) [PDF], Amgen, March 2019.
3. Summary of Product Characteristics [FDA]: AIMOVIGTM (erenumab) [PDF], Amgen, May 2018.
4. GRP as the target of new migraine therapies. Edvinsson L. et al. Nature Reviews Neurology. 24/04/2018; volume 14: 338–350.
5. New Zealand Data Sheet (Medsafe): AIMOVIGTM (erenumab) [PDF], Novartis, January 2020.
6. OCmigraine.org: Difference between Aimovig, Ajovy, and Emgality
7. Summary of Product Characteristics [FDA]: VYEPTI (eptinezumab-jjmr) [PDF], Lundbeck, February 2020
Disclaimer: This article is not meant to influence or impact the care provided by your treating physician. Please do not make changes to your treatment without first consulting your healthcare provider. This article is not intended to diagnose or treat illness or to influence treatment options. everyone.org is as diligent as possible in compiling and updating the information on this page. However, everyone.org does not guarantee the correctness and completeness of the information provided on this page.