What is Tudca? | Information & Faq
Last updated: 02 July 2024

You can legally access new medicines, even if they are not approved in your country.
Learn howArticle reviewed by Dr. Jan de Witt
Article last updated on 21/1/2021
If you’ve heard about TUDCA, you might have some questions about what it means for patients with ALS and other diseases. Here are 5 common questions about TUDCA, with easy-to-understand answers.
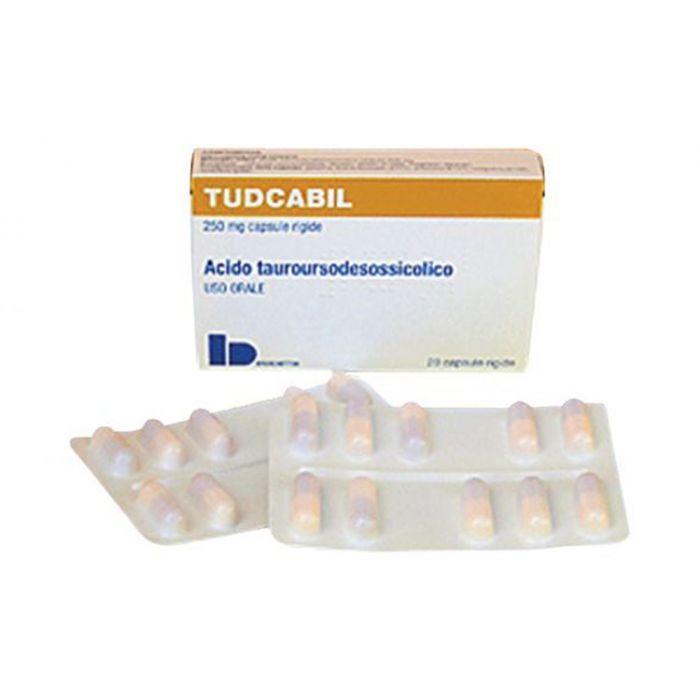
TUDCA (which has the trade name Tudcabil) is currently classified as a ‘supplement’ for certain liver disorders but has shown to be useful in treating amyotrophic lateral sclerosis (ALS), Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease.
Due to the fact that studies with TUDCA are still ongoing, it is not approved in all countries and therefore is not available to patients and healthcare professionals globally. However, there are options for individuals and doctors to import medicines for personal use, even in countries where a medicine may not be available. Our team supports doctors and patients and delivers unapproved medicines globally on daily basis. Find more information on our home page about how to access and import medicines not yet approved in your country.
1. What is TUDCA?
Tauroursodeoxycholic acid (TUDCA) is a compound originally approved to treat liver diseases — but recent research suggests it may have a broader application.
TUDCA is closely related to ursodeoxycholic acid (UDCA), with some structural differences. TUDCA is one of the compounds found in bile acid. Bile acid is made by the liver and stored in the gallbladder and helps with digestion.
Recently, TUDCA is being used and tested in a wide variety of therapeutic areas to explore its effectiveness. Amongst others, TUDCA has been studied for the way in which it may relieve the symptoms of inflammatory diseases.
For more information about Tudca and how we can help you access it, click on the button below:
2. Where does TUDCA come from?
Interestingly, TUDCA has been used in Chinese traditional medicine for centuries to treat a range of ailments. In Chinese medicine, it was originally harvested from the bile of bears. Bear bile is made up of more than 50% TUDCA. Some scientists believe this is what gives bears the ability to hibernate for extended periods of time.
TUDCA is now manufactured as a biopharmaceutical. Tudcabil (TUDCA) is available in packs of 20 capsules, each 250mg.
3. How does TUDCA work?
TUDCA is already used to treat liver disease and much of the information available about TUDCA’s effects comes from studying liver cells. It is not verified exactly how TUDCA works, but it is thought that it works to prevent programmed cell death (also known as apoptosis).
ALS (MND) involves the gradual deterioration of nerve cells. In the same way as TUDCA prevents cells in the liver from dying, it is thought that it may slow down the progression of cell death in ALS patients, although clinical trials for TUDCA on ALS are still ongoing.
There are also studies to suggest that TUDCA may be useful in treating the following diseases, although studies are still ongoing and it is not formally approved to treat these diseases as yet:
- Alzheimer’s Disease
- Parkinson’s Disease
- Huntington’s Disease
- ocular disorders
- strokes
- obesity
- cardiovascular disorders
- gastrointestinal disorders
- renal injury.
4. Is TUDCA approved?
TUDCA is approved in Italy for the treatment of liver disorders. In addition it has recently been granted the status of ‘orphan designation’ by the European Medicines Agency (EMA).
Orphan designation is given to medicines if they qualify for three criteria: if they are used to treat a very serious condition; if there is a lack of alternative medicines to treat the disease/condition; and if the condition is rare, or there will be little return on investment for the manufacturer of the medicine (because the patient group is small).
5. Is TUDCA available to me?
TUDCA is not approved in many countries and therefore might not be available in many countries outside of Italy.
Most countries, however, have regulations that allow for personal importation of medicines on a ‘named patient basis’ by either an individual or a healthcare professional. Though this can only be done according to the local regulations and with a doctor’s prescription.
everyone.org has the experience to guide you through this process. Right now, we have daily deliveries of elsewhere-approved medicines around the world, with service that’s highly rated by doctors and patients. Your medicine can be with you in your home country within one or two weeks. You can find information on our home page about how we can assist you and your doctor with this as well.