KRAS inhibitors for lung cancer: All about the new treatments bringing hope
Last updated: 15 January 2024

You can legally access new medicines, even if they are not approved in your country.
Learn howIf you or someone close to you are a lung cancer patient, you're probably already familiar with the term KRAS. Whether that's the case or you're new to the topic, you're in the right place.
In this article, we'll explain everything you need to know about KRAS mutations and KRAS inhibitors., including how they work and how they're changing the landscape of lung cancer treatments.
The KRAS oncogene and its mutations
KRAS, short for Kirsten rat sarcoma viral oncogene homolog. Like other oncogenes, KRAS has the ability to transform cells into cancer cells, under specific circumstances [1]. These circumstances are often related to mutations of the oncogene - i.e. KRAS mutations.
The unchanged, natural form of KRAS is known as wild-type KRAS. It makes a protein that signals to cells that they should grow, multiply, or die.
When a KRAS mutation occurs, the protein produced by the KRAS oncogene becomes overactive. It sends signals to cells, causing them to grow and spread in the body [2].
Types of KRAS mutations in lung cancer
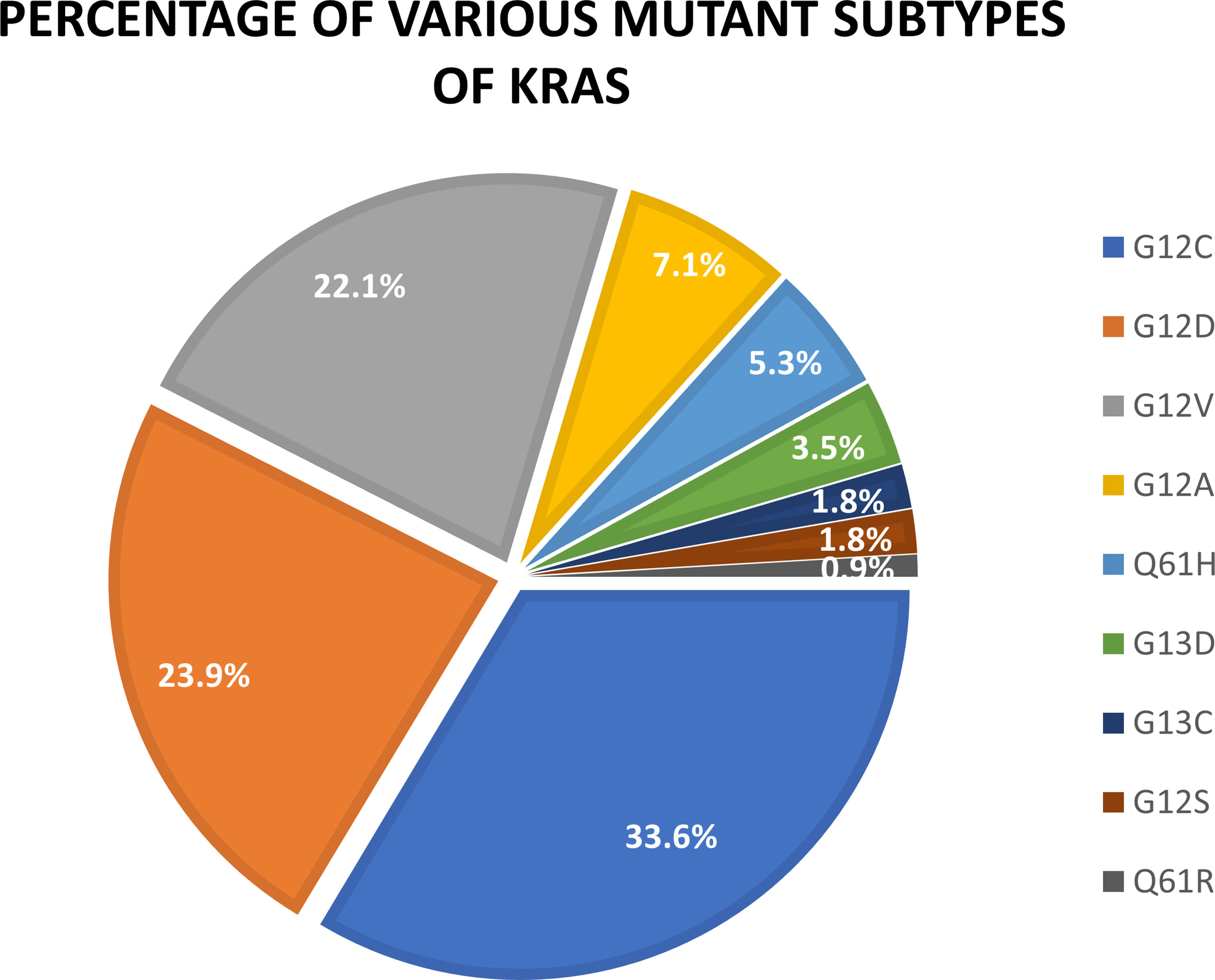
KRAS mutations occur in 20-40% of all lung cancers [4]. There are multiple types of KRAS mutations. However, when it comes to lung cancer, the most common mutations are KRAS G12C, G12D, and G12V. They account for 33.6%, 23.9%, and 22.1% of total KRAS mutations, respectively [3].

Standard treatments for KRAS-mutated lung cancer
The first line of treatment for lung cancer is the same for patients with and without a KRAS mutation. Standard courses of treatment include surgery, radiation, chemotherapy, immunotherapy, or a combination of these [5].
Does chemo work on KRAS mutations?
For some patients, standard therapies can deliver positive results. Outcomes vary from one patient to another. Some studies show that immunotherapy may be more efficient than chemotherapy against KRAS-mutated NSCLC. In fact, KRAS-mutant non-small cell lung cancers (NSCLC) seem to respond to immunotherapy as well as non-KRAS-mutant NSCLC [6].
But what about the patients who have no (sufficient) success with the standard courses of treatment? Up until 2021, there were no other second-line treatment options available for NSCLC patients with KRAS mutations.
Now, there is a new type of treatment. Enter KRAS inhibitors.
KRAS Inhibitors: No longer a pipe dream
For decades, direct targeting of KRAS mutant proteins has been a challenging task for cancer researchers. Partially, due to the nature of KRAS. Its small and shallow surface makes it difficult for small molecules to bind to it [7]. In addition, each type of KRAS mutation requires a different approach. What works on KRAS G12C won't necessarily work on KRAS G12D or KRAS G12V.
Through the years, indirect KRAS inhibitors have been explored. Some have tried interrupting the KRAS membrane location. Others have tried interfering with the interaction between KRAS and its effector proteins. All these indirect approaches have had underwhelming results [7].
That's why having the first approved direct KRAS inhibitors is the biggest recent breakthrough in KRAS-mutant NSCLC treatment.


KRAS Inhibitors for KRAS G12C
Due to its dynamic nucleotide cycling mechanism, KRAS switches rapidly between an "active" and "inactive" state - i.e. a state when it sends growth signals to cells or a state when it doesn't. When the KRAS G12C mutation occurs, KRAS gets "stuck" in an active state of signaling [8].
What researchers have long aimed to achieve with KRAS G12C inhibitors is to stabilize KRAS in its inactive state.
Currently, there are two approved KRAS G12C inhibitors which achieve that. One is Sotorasib (also known as Lumakras) and the other - Adagrasib (also known as Krazati).
Sotorasib (Lumakras)
Since its FDA approval in 2021, Sotorasib has been approved in many other regions throughout the world. It's used as second-line therapy for patients with advanced non-small cell lung cancer with the KRAS G12C mutation.
Sotorasib is also being tested on patients with other types of cancer with the KRAS G12C mutation - e.g. pancreatic or colorectal cancer. More research is needed to understand the potential of Sotorasib in treating other types of cancer.
If Sotorasib is not approved in your country, you can still access it if you have a prescription. Find out how we can help you access Sotorasib.
Adagrasib (Krazati)
Adagrasib is a newer drug than Sotorasib. It has only been approved in the USA so far, but its initial results are quite promising.
Similar to Sotorasib, Adagrasib is used in patients with advanced NSCLC, who have seen insufficient results with standard therapies such as chemotherapy or immunotherapy.
There are some small differences between Sotorasib and Adagrasib. However, both KRAS G12C inhibitors work in a similar way overall.
If Adagrasib is not approved in your country, you can still access it if you have a prescription. Find out how we can help you access Adagrasib.
KRAS Inhibitors for KRAS G12D
There is currently no approved KRAS inhibitor targeting the KRAS G12D mutation specifically. However, there are several inhibitors in development, including TH-Z835, TH-Z827, KD-8, and MRTX1133 [11]. Some of them are in very early development stages. MRTX1133 is the most promising KRAS G12D inhibitor in development. It has been cleared for Phase 1 clinical trials [10].
Pan-KRAS inhibitors
A new type of KRAS inhibitor is also currently being developed. Known as pan-KRAS inhibitors, these drugs aim to target multiple KRAS mutations at once [12]. Pan-KRAS inhibitors in development include BI 1701963 and RMC-6236. BI 1701963's potential use in combination with Lumakras (sotorasib) is currently also being studied [13].
KRAS Inhibitor Resistance
KRAS inhibitors show positive clinical results. Unfortunately, cancer cells eventually develop resistance mechanisms to these targeted therapies. For example, by further KRAS mutations that stop the drug from properly binding with the KRAS molecule.
As a result, even patients whose tumors have stabilized or shrunk from KRAS inhibitors, are likely to experience disease progression after some time [14].
Researchers are looking at different ways to tackle KRAS inhibitor resistance. Some of the approaches are combination therapy and alternating the use of different inhibitors [15]. Pan-KRAS inhibitors may also play a role in solving the KRAS inhibitor resistance challenge. However, more data are needed to determine the best approach.
Final words on KRAS inhibitors
The challenges of treating KRAS mutations in lung cancer are far from over. However, for advanced patients with limited treatment options, KRAS inhibitors open a whole new avenue of hope.
With KRAS inhibitors and pan-KRAS inhibitors gaining ground, we hope to see more treatment options available to advanced NSCLC patients. As soon as new NSCLC therapies are approved anywhere in the world, Everyone.org can help you access them everywhere. Get in touch with us to find out more.
References:
- Definition of oncogene - NCI Dictionary of Cancer Terms - NCI. National Cancer Institute, Accessed 16 June 2023.
- Definition of KRAS gene - NCI Dictionary of Cancer Terms - NCI. National Cancer Institute, Accessed 16 June 2023.
- Characterization With KRAS Mutant Is a Critical Determinant in Immunotherapy and Other Multiple Therapies for Non-Small Cell Lung Cancer. Frontiers, 2 December 2021.
- KRAS-mutant non-small cell lung cancer: Converging small molecules and immune checkpoint inhibition. NCBI, 7 March 2019.
- KRAS and Lung Cancer. American Lung Association, 17 November 2022.
- Van Geffen, Wouter H. Efficacy of immunotherapy in KRAS-mutant advanced NSCLC: A real-world study in a Chinese population. Frontiers, 19 January 2023.
- KRAS mutation: from undruggable to druggable in cancer. Signal Transduction and Targeted Therapy, 15 November 2021.
- KRAS-Targeted Therapy Doubles as Component of Immunotherapy. National Cancer Institute, 31 October 2022.
- Tucker, Nichole. Treatment With Novel KRAS Inhibitor Commences in Phase 1/1B Study, COVALENT-10. Targeted Oncology, 19 January 2023.
- Mirati Announces IND Clearance by U.S. FDA Enabling Phase 1 Initiation for First-in-Class Oral KRASG12D Selective Inhibitor, MRTX1133. PR Newswire, 19 January 2023.
- KRAS G12D targeted therapies for pancreatic cancer: Has the fortress been conquered?. Frontiers, 8 November 2022.
- Expanding the Reach of Precision Oncology by Drugging All KRAS Mutants. Cancer Discovert, 01 April 2022.
- BI Enters Collaboration with Amgen. Boehringer Ingelheim, 16 September 2021.
- Resistance to KRASG12C Inhibitors in Non-Small Cell Lung Cancer. NCBI, 24 December 2021.
- Next Steps for KRAS Inhibitors Focus on Tackling Resistance. OncLive, 17 January 2022




