What's the difference between Sotorasib and Adagrasib? A simple overview.
Last updated: 15 January 2024

You can legally access new medicines, even if they are not approved in your country.
Learn howSotorasib (marketed as Lumakras) was the first targeted therapy for NSCLC with the KRAS KG12C mutation. Its FDA approval in 2021 was considered by many to be a breakthrough for patients with advanced non-small cell lung cancer. It also paved the way for the approval of other similar drugs, such as Adagrasib (marketed as Krazati).
If you're wondering what is the difference between Sotorasib and Adagrasib, you're not alone.
In this article, we'll compare the two drugs on the way they work, their reported success rate, as well as pricing and availability.
Sotorasib vs Adagrasib: What are they used for?
Based on their reported clinical trial outcomes, Sotorasib and Adagrasib are considered promising drugs in the treatment of non-small cell lung cancer (NSCLC), which accounts for about 85% of all lung cancer cases.[12,13]
Both drugs are targeted therapies. They're designed to target and inhibit the activity of the KRAS G12C mutation. This mutation is prevalent in approximately 13% of patients with non-small cell lung cancer.
Sotorasib and Adagrasib are intended for patients with advanced KRAS G12C-mutated NSCLC that has spread to other organs. Both drugs are used in patients who have received at least one round of prior cancer treatment.
Sotorasib vs Adagrasib: How do they work?
Similarities
Both drugs are covalent KRAS G12C inhibitors. In other words, they bind with the KRAS G12C protein and inactivate it. By doing so, they prevent the protein from signaling to tumor cells to grow and multiply. According to clinical reports, this results in tumor shrinkage and improved clinical outcomes for patients.
Differences
One of the differences between the two drugs is the part of the KRAS G12C protein they bind to. Sotorasib binds to the cysteine of KRAS G12C [1], whereas Adagrasib binds directly to the amono acid [2].
Another difference, according to the Journal of Clinical Oncology, is the ability of Adagrasib to penetrate the central nervous system and slow down the growth of cancer cells in the brain [11]. There are currently no data to show the same effect for Sotorasib.
Despite these slight differences in the way the two drugs work, they have the same goal and effect - stopping the growth of cancer cells.
Are Sotorasib and Adagrasib chemotherapy?
No, neither of the two drugs is chemotherapy. Both Sotorasib and Adagrasib are KRAS G12C inhibitors.
Sotorasib vs Adagrasib: Reported Efficiency
The efficiency of the two drugs has been evaluated in clinical trials.
Note that the clinical trials for Sotorasib took place before those for Adagrasib. As a result, we already have the 2-year follow-up data for Sotorasib trial participants. In the following comparison, we will look at the 2-year reported data for Sotorasib [3] and the most recent reported data available for Adagrasib [4].
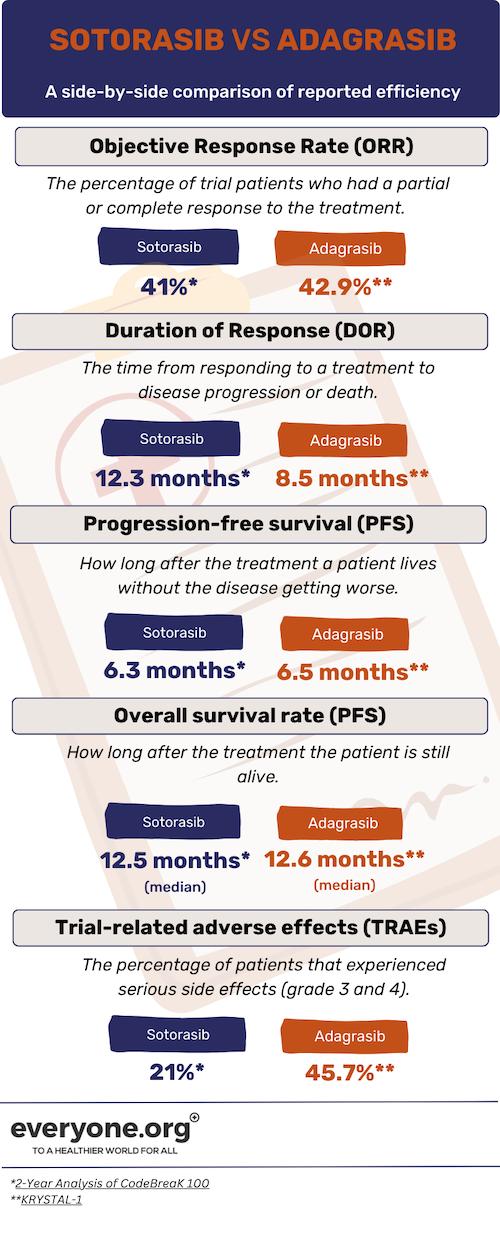

Both Sotorasib and Adagrasib show potential in treating NSCLC patients with KRAS G12C mutations. Based on the data we have, the main difference between the two drugs appears to be the duration of response, with Sotorasib showing a better result according to the reports. Additionally, the reported data show noticeable differences in the number of patients experiencing serious side effects.
The results shared here are for informational purposes and should not be used as the basis of a treatment choice. Your doctor is in the best position to determine the treatment that best applies to your case.
Sotorasib vs Adagrasib: Safety and side effects
According to the reports, both Sotorasib and Adagrasib have similar side effects for the most part. These include:
- Stomach and intestinal problems, including nausea, diarrhea, or vomiting. Adagrasib can also cause intestinal bleeding, colitis and stenosis.
- Liver problems. Both drugs can cause abnormal liver blood test results, as well as serious side effects such as treatment-induced hepatitis, jaundice, changes in the coloration of urine and stools, loss of appetite.
- Lung or breathing problems: both drugs may cause life-threatening lung inflammations.
There is one side effect documented for Adagrasib but not for Sotorasib. It's related to changes in the hearts' electrical activity (QTc prolongation). According to the reports, this condition can contribute to life-threatening irregular heartbeats, including torsades de pointes [5].
Do Sotorasib and Adagrasib work for pancreatic cancer?
KRAS mutations occur in 44.4% of gastrointestinal cancers, including pancreatic cancer. About 4% of these are G12C-type mutations [7].
Sotorasib and Adagrasib are reported to show promising results in targeting KRAS G12C mutations in lung cancer. But can they do the same for pancreatic and other gastrointestinal cancers?
The short answer is probably, but we need more data.
In preclinical studies, both drugs are reported to have promising efficacy in patients with advanced pancreatic and gastrointestinal tumors. Sotorasib has a reported objective response rate (ORR) of 21% among advanced pancreatic cancer patients [8]. Adagrasib has a similar reported ORR of 22% among patients with advanced colorectal cancer [9].
These early findings seem to be positive. However, more research is needed to understand the potential role of Sotorasib and Adagrasib in treating pancreatic and gastrointestinal cancers. Both drugs reportedly have limitations, such as resistance development or side effects, which require further study in clinical trials.
Sotorasib vs Adagrasib: Price comparison
It's difficult to compare the two drugs in price. The actual costs may vary depending on suppliers, your location, and insurance coverage.
However, an indicative price for a 30-day supply is approximately EUR 12,500 for Sotorasib (Lumakras), and approximately EUR 19,400 for Adagrasib (Krazati) [10].
Where are Sotorasib and Adagrasib approved?
Sotorasib is currently approved as a prescription medicine for KRAS G12C-mutated NSCLC in the USA, EU, UAE, Canada, UK, and Japan.
Adagrasib is currently only approved in the USA.
Is Sotorasib or Adagrasib not approved or available in your country? We can help you access these medicines if you have a prescription from your doctor.
Contact us, so we can give you a detailed price quote for sourcing the medicines for you. Or have a look at the medicine pages below for more information.
References:
-
Lee, Arnold. Sotorasib: A Review in KRAS G12C Mutation-Positive Non-small Cell Lung Cancer. NCBI, 2 December 2022.
- Definition of Adagrasib - NCI Drug Dictionary - NCI. National Cancer Institute, Accessed 30 May 2023.
-
Grace, K. Dy. Long-Term Outcomes and Molecular Correlates of Sotorasib Efficacy in Patients With Pretreated KRAS G12C-Mutated Non–Small-Cell Lung Cancer: 2-Year Analysis of CodeBreaK 100. Journal of Clinical Oncology, 25 April 2023.
- Spira, Alexander. KRYSTAL-1: Activity and safety of adagrasib (MRTX849) in patients with advanced/metastatic non–small cell lung cancer (NSCLC) harboring a KRASG12C mutation. Journal of Clinical Oncology, 02 June 2022.
- KRAZATI USPI. Mirati Therapeutics. Accessed 30 May 2023.
- Adagrasib Penetrates CNS in Early Data of KRAS G12C+ NSCLC. Targeted Oncology, 7 June 2022.
- Characterisation of KRAS, Including KRAS G12C, Mutations in Gastrointestinal and Metastatic Colorectal Cancer. European Society for Medical Oncology, 2 July 2021.
- LUMAKRAS® (SOTORASIB) SHOWS ENCOURAGING AND CLINICALLY MEANINGFUL ANTICANCER ACTIVITY IN PATIENTS WITH KRAS G12C-MUTATED ADVANCED PANCREATIC CANCER IN CODEBREAK 100 TRIAL. Amgen, 15 February 2022.
-
Weiss, J. LBA6 KRYSTAL-1: Adagrasib (MRTX849) as monotherapy or combined with cetuximab (Cetux) in patients (Pts) with colorectal cancer (CRC) harboring a KRASG12C mutation. Colorectal Cancer, September 2021.
- Krazati Prices, Coupons, Copay & Patient Assistance. Drugs.com, Accessed 30 May 2023.
- Activity of adagrasib (MRTX849) in patients with KRASG12C-mutated NSCLC and active, untreated CNS metastases in the KRYSTAL-1 trial. Journal of Clinical Oncology, accessed 16 June 2023.
- Adagrasib Induces Promising Efficacy, Deep Responses in KRAS G12C NSCLC. Cancer Network, 03 June 2022.
- Li, Bob. 2-Year Follow-Up Shows Sotorasib Significantly Prolongs Survival in Patients With Non-Small Cell Lung Cancer. Roswell Park Comprehensive Cancer Center, 26 April 2023.




