Renal cell carcinoma (RCC) also called kidney cancer or renal cell cancer is a disease in which malignant cancer cells are found in the lining of tubules in the kidney. RCC accounts for more than 80% of all kidney cancers.

Renal cell carcinoma (RCC) also called kidney cancer or renal cell cancer is a disease in which malignant cancer cells are found in the lining of tubules in the kidney. RCC accounts for more than 80% of all kidney cancers.

Rheumatoid arthritis (RA) is an autoimmune disease where the immune system attacks body tissues. It primarily affects joints, but it can also cause inflammation of organs, such as the lungs, eyes, skin, and heart.

Multiple Sclerosis (MS) is a chronic, inflammatory, autoimmune disease of the central nervous system that disrupts communication between the brain and other parts of the body.

Parkinson’s disease (PD) is a progressive brain disorder that causes shaking and muscle stiffness, and slows movement.

Colorectal cancer or colon cancer is cancer of the large intestine (colon), which is the final part of the digestive tract. Both colon cancer and rectal cancer can be referred to as colorectal or bowel cancer, but there is a slight difference between the two based on where the cancer begins.

There are several ALS medications that are not yet approved in any country and currently under clinical trial. Read about the latest approved treatments.

Human epidermal growth factor receptor 2 (HER2) is a growth-promoting protein on the outside of all breast cells. Breast cancer cells with higher than normal levels of HER2 are called HER2-positive.

Triple-negative breast cancer (TNBC) is a cancer that tests negative for progesterone and estrogen receptors, and excess HER2 protein. This means that the growth of the cancer is not fuelled by the estrogen and progesterone hormones, or by the HER2 protein. So, TNBC does not respond to hormonal therapy medicines or medicines that target HER2 protein receptors.

Aimovig (erenumab) is a preventive migraine treatment that works by blocking the activity of calcitonin gene-related peptide, a molecule that is involved in migraine attacks

Here is an overview of each medicine to help guide you and your physician when making a treatment decision for Cystic Fibrosis: 
Here are some frequently asked questions and answers that touch on important COVID-19 related updates.
5 common questions about the supplement that may be useful for ALS, Parkinson's and Alzheimers.

New analysis of Biogen’s aducanumab shows reduction in clinical decline in Alzheimer’s disease.
Kyowa Hakko Kirin's Parkinson's therapy, Nourianz (istradefylline), gains FDA approval as an add-on to levodopa/carbidopa.

Radicut/Radicava (edaravone), shown to slow the progression of ALS, is now approved for patients in China.

Orkambi and Symkevi have been rejected by the Scotland Medicines Consortium (SMC) due to their conclusion that the costs outweigh the benefits.

Vitrakvi (larotrectinib) recommended by the CHMP for approval in the EU.

MN-166 (ibudilast) will be part of the Phase III clinical trial on progressive multiple sclerosis subjects with secondary progressive MS without relapses.

New skin cancer medicine now approved in the EU for adults with metastatic or locally advanced cutaneous squamous cell carcinoma.

Phase 2 clinical trial shows that ibudilast is linked to a 48% slowing in the progression of brain atrophy compared to placebo for patients with progressive MS.
Vyndaqel (tafamidis) is now approved in the U.S. after study showed increased survivorship rate and reduced hospital time due to heart related problems.

Approval for next phase of clinical trials puts ALS patients one step closer to accessing new treatment shown to slow disease progression.
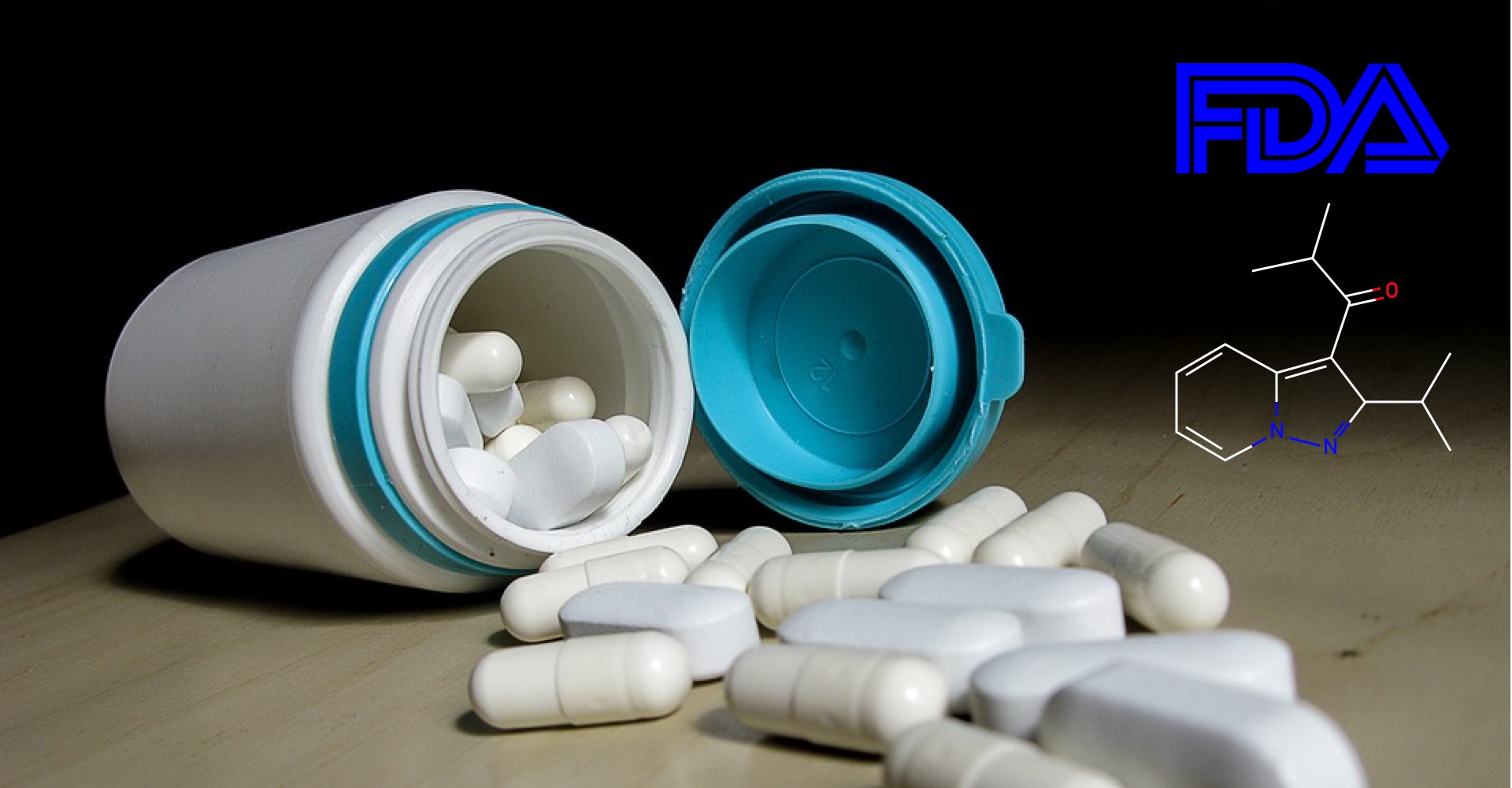
Based on promising results from phase III clinical trials, Health Canada has decided to extend the use of Kalydeco (ivacaftor) to include children aged 1 to 2 years.

A recent clinical trial indicates that Symkevi/Symdeko (tezacaftor/ivacaftor) can safely and effectively loosen thick, sticky mucus for children 6-11 years old.

The new site-agnostic cancer medicine, Vitrakvi (larotrectinib), gets accelerated approval from the U.S. Food and Drug Administration (FDA).

CHMP's assessment supports EMA's approval of non-small cell lung cancer (NSCLC) medicine, Vizimpro (dacomitinib).

Phase III trial shows that Copiktra (duvelisib) may be an option for patients with relapsed or refractory chronic lymphocytic leukaemia and small lymphocytic lymphoma.

Onpattro (patisiran) is a first-in-class treatment that slows down the progression of hereditary transthyretin amyloidosis (hATTR).

After failing to show a clear benefit over chemotherapy on its own, both the FDA and EMA do not recommend Lartruvo (olaratumab) for use in combination with chemotherapy for new STS patients.

Over 50 countries have approved Spinraza (nusinersen), but not all have made it available to the patients who need it.
