Your Complete Guide to Leqembi: An Alzheimer's Drug Worth Remembering
Last updated: 19 November 2024

You can legally access new medicines, even if they are not approved in your country.
Learn howIn January 2023, the FDA granted accelerated approval to a new Alzheimer’s medication under the name of Leqembi (lecanemab). This came less than a year after the accelerated approval of another similar drug - Aduhelm. Although Aduhelm has had its share of controversy [1], the introduction of both treatment options is expected to provide a glimpse of hope for Alzheimer’s patients worldwide.
Here’s everything you need to know about Leqembi.
What is Leqembi (lecanemab)?
Leqembi (lecanemab) is an amyloid-beta directed antibody therapy. It's designed to treat adult patients with early Alzheimer's, who have mild cognitive impairment or dementia. According to Leqembi's prescribing information, the drug is appropriate for patients with confirmed elevated beta-amyloid levels[2].
What is mild cognitive impairment?
Mild cognitive impairment (MCI) is characterized by a decline in an individual's memory and cognitive ability, which exceeds the normal decline expected for their age or educational level. Common symptoms of MCI include forgetfulness, difficulty remembering words, and problems with decision-making.
Not all people with MCI develop Alzheimer's. However, it's been reported that 10-20% of adults over the age of 65 who have MCI progress to develop Alzheimer's within a year [3].
How does Leqembi work?
Studies show that one of the factors contributing to Alzheimer’s progression, is the buildup of amyloid beta plaques in the brain. These plaques disrupt and, eventually, destroy neurons.
As a monoclonal antibody, Leqembi (lecanemab) targets and intends to remove amyloid beta plaques in the patient’s brain. This intends to help slow down cognitive decline [3].
Is Leqembi a cure for Alzheimer's disease?
No, Leqembi (lecanemab) isn't a cure for Alzheimer's. A cure for Alzheimer’s unfortunately does not yet exist. However, Leqembi intends to help slow down the progression of the disease. This might allow people diagnosed with Alzheimer’s to enjoy a good quality of life for a longer period of time.
Can Leqembi restore lost memories or cognitive functions?
No. There's currently no evidence to suggest that Leqembi (lecanemab) can reverse memory loss or loss of other cognitive functions [4].
Does Leqembi (lecanemab) work for younger patients?
Alzheimer’s disease is most prevalent in the population aged 65 and above. However, some younger individuals may also be at risk of developing symptoms. This especially applies to those with high levels of brain amyloid [8].
Lecanemab has not been tested for safety or efficacy in younger and/or pre-symptomatic patients. However, there are some lecanemab studies currently enrolling participants as young as 55.
One example is the global AHEAD study. It aims to examine the effects of lecanemab if applied before the onset of significant brain damage [9]. The results of these studies will in the future shed more light over the potential use of Leqembi in younger patients.


How is Leqembi different from other Alzheimer’s medicines?
Until recently, Alzheimer’s medication options were limited to mostly symptomatic treatments. For example, galantamine, rivastigmine, and donepezil. These medicines are known as acetylcholinesterase inhibitors. They support treatment by preventing the breakdown of acetylcholine - a brain chemical involved in memory and other essential cognitive functions [10].
Unlike acetylcholinesterase inhibitors, Leqembi is not a symptomatic drug. Instead, it addresses the underlying disease process and aims to slow down disease progression.
Although there are some differences between Leqembi and Aduhelm, both drugs work in a similar way overall.
How effective is Leqembi?
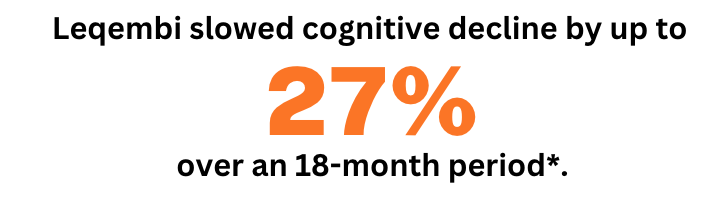
Clinical trial reports by Leqembi's manufacturer, Eisai, claim that Leqembi can slow down the cognitive decline of Alzheimer’s patients by as much as 27%, compared to the placebo group. These results were measured in patients with mild dementia, who received the lecanemab treatment over an 18-month period [5].
Based on these reported data, Leqembi seems to provide a glimpse of hope for changing the course of the disease for early-stage patients.
However, members of the FDA are still debating whether the benefits of the drug for patients are large enough to justify its full approval [6]. Additional data coming from further research and real-life patients will hopefully shed more light on this issue in the near future.

Potential side effects of Leqembi (lecanemab)
Leqembi’s most common side effects include:
- Infusion-related reactions
- Headaches
- Amyloid Related Imaging Abnormalities (ARIA) [2].
Infusion-related reactions
These may include fever, flu-like symptoms, nausea, changes in heart rate, and shortness of breath. 26.4% of clinical trial participants had these reactions to their lecanemab treatment [5].
Amyloid Related Imaging Abnormalities (ARIA)
ARIA is a reaction to lecanemab, which may involve temporary swelling or bleeding in the brain. Symptoms include headaches, confusion, dizziness, difficulty walking, and seizures.
Some people may be at a higher risk for ARIA, particularly carriers of the homozygous apolipoprotein E gene [7].
3% of patients in Clinical Study 1 showed ARIA symptoms. For 80% of these patients, symptoms resolved within the duration of the study [2].
Safety during pregnancy and breastfeeding
Clinical trials conducted so far did not focus on the effects of Leqembi during pregnancy and/or breastfeeding. It is unknown if lecanemab passes into breast milk.
For a full overview of potential side effects, please refer to the Medication guide [7].
How to take Leqembi (lecanemab)
Patients receive Leqembi as an intravenous infusion in the arm, at a dose of 10 mg/kg of body weight. Each infusion lasts about 1 hour. Most commonly, the treatment repeats at a 2-week Interval [2].
How does Leqembi interact with other medicines?
One of Leqembi’s known potential side effects is ARIA, which is related to bleeding in the brain. If you’re taking blood-thinning medication, including aspirin, this may increase the risk of ARIA. It’s important to thoroughly discuss this with your doctor prior to starting the treatment [2].
How much does Leqembi cost?
It's difficult to provide the exact cost of Leqembi. The final price tag may vary depending on your location, the supplier, and other factors, such as insurance coverage.
As an indication, a single dose of Leqembi costs approximately EUR 1,043. The recommended frequency of treatment is once per two weeks. This brings the yearly costs of treatment for 1 person to approximately EUR 27,118.
To get a personalized quote for the medicine, make a request via the Leqembi overview page.
Where is Leqembi approved?
As of November 2024, Leqembi is approved by the FDA in the USA, the MHRA in the UK, and it's expected to be officially approved by the EMA by the end of 2024.
The developer of the therapy, Eisai, is also seeking approval for Leqembi in China [13] and Japan [14].
Even if Leqembi is not approved or available in your country yet, you can still access it via Everyone.org, if you have a prescription from your doctor. So first consult your doctor to weigh up the pros and cons. If you and your doctor agree Leqembi might be the best treatment option for you, get in touch with our access experts to find out how to get Leqembi outside the USA.
REFERENCES:
- Alzheimer's disease. NHS, Accessed 26 June 2023.
- LEQEMBI Prescribing Information. Leqembi, Accessed 26 June 2023.
- What Is Mild Cognitive Impairment? | National Institute on Aging. National Institute on Aging, 12 April 2021.
- Lecanemab Approved for Treatment of Early Alzheimer's Disease. Alzheimer's Association, Accessed 26 June 2023.
- Lecanemab in Early Alzheimer’s Disease. The New England Journal of Medicine, 05 January 2023.
- McGinley, Laurie. Alzheimer's drug Leqembi backed by FDA advisers for full approval. The Washington Post, 9 June 2023.
- LEQEMBI Medication Guide. Leqembi, Accessed 26 June 2023.
- Donohue, Michael C. Association Between Elevated Brain Amyloid and Subsequent Cognitive Decline Among Cognitively Normal Persons. NCBI, 13 June 2017.
- Macmillan, Carrie. Lecanemab, the New Alzheimer's Treatment: 3 Things To Know. Yale Medicine, 19 January 2023.
- How Is Alzheimer's Disease Treated? | National Institute on Aging. National Institute on Aging, 22 June 2023.
- Maker of promising Alzheimer's drug Leqembi expects full FDA approval this summer, expanded Medicare coverage. CNBC, 17 February 2023.
- EISAI SUBMITS MARKETING AUTHORIZATION APPLICATION FOR LECANEMAB AS TREATMENT FOR EARLY ALZHEIMER'S DISEASE IN EUROPE | Biogen. Biogen | Investor Relations, 10 January 2023.
- Eisai Initiates BLA Submission of Data for Lecanemab in China | Biogen. Biogen | Investor Relations, 22 December 2022.
- Carvalho, Teresa, and Ray Burow. Lecanemab, for Early Alzheimer's Coming Up for Review in Japan. Alzheimer's News Today, 14 March 2022.