
Vyndaqel (tafamidis): Amyloidosis treatment approved by the FDA
Transthyretin amyloidosis is a rare hereditary disease in which a fibrous substance called amyloid is deposited in tissues and organs around the body, including the nerves. These deposits interfere with the normal nerve function and can result in a condition called polyneuropathy, characterised by a sense of weakness, numbness, and pain in the hands and feet. Tafamidis, also known by the brand name Vyndaqel, is used to slow down nerve damage in patients with early-stage polyneuropathy.
Tafamidis (Vyndaqel) is used to treat a rare disease known as transthyretin familial amyloid polyneuropathy (TTR-FAP). It has been approved in many countries, including Brazil, Israel, South Korea, those in the European Union, and in the United States. In some other countries, e.g. Australia, it has orphan designation* for the treatment of transthyretin cardiomyopathy (TTR-CM)–in which the disease affects the heart muscle.
* With the orphan drug designations, the evaluation and development of this medicine can be done more quickly, in the hope that people with these serious and rare conditions will be able to benefit from new treatment options that would otherwise take longer to become available, if at all.
Tafamidis is the active ingredient in Vyndaqel which comes in capsule form. It is indicated for patients affected by transthyretin amyloidosis who also suffer from polyneuropathy. Polyneuropathies, or peripheral neuropathies, result from damage to the peripheral nerves (nerves that go from the spinal cord to the arms, hands, legs, and feet), which often leads to a sense of weakness, numbness, and pain in the extremities.
Learn more about costs and delivery
The efficacy of tafamidis (Vyndaqel) has been tested in clinical trials. The main study lasted 18 months and included 128 patients carrying a specific genetic mutation (V30M). These participants were in stage 1 of the disease, meaning they were able to walk without support most of the time. Those taking tafamidis (Vyndaqel) showed results superior to the placebo, with less nerve damage and a higher quality of life.
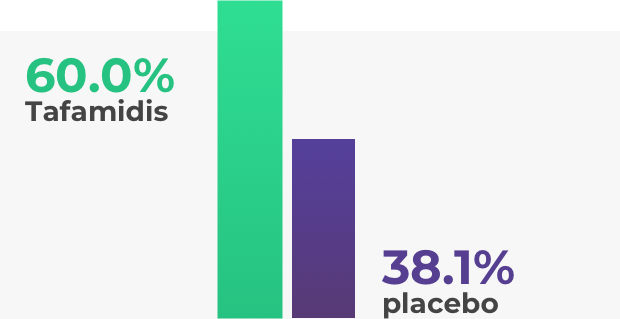
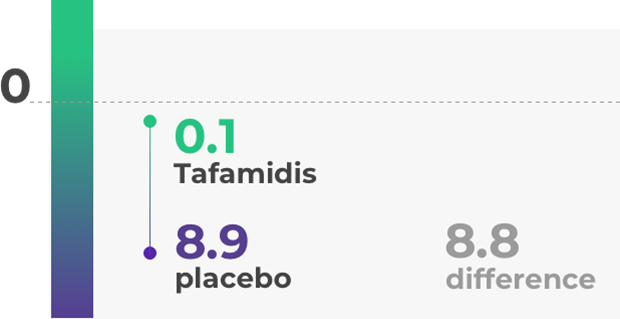
For more details refer to the prescribing information at the bottom of the page.
The most common side effects listed in the prescribing information include diarrhoea, urinary tract infection, stomach ache or abdominal pain, and vaginal infection in women.
Please note this is not intended to be a comprehensive guide. Consult your treating doctor and the prescribing information at the bottom of the page for full details of side effects.
The capsules should be taken orally, once daily. They should be swallowed whole, not cut or crushed, and can be taken with or without food.
Please note this is not intended to be a treatment plan. For a personalised treatment plan consult your doctor. For more details, you can also reference the full prescribing information at the bottom of the page.
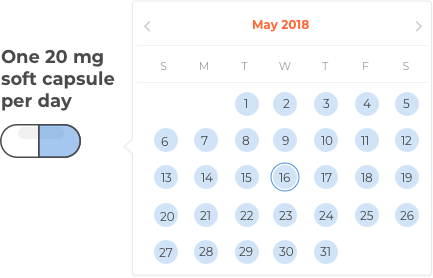




For more details about the way tafamidis (Vyndaqel) works, consult your doctor or see the prescribing information at the bottom of the page. This is not intended to be a comprehensive scientific explanation.

If you or one of your relatives or friends are a patient with transthyretin amyloidosis, you might want to ask questions about the medicine for this disease. If you’d like to ask questions about how to get tafamidis (Vyndaqel), or about our service in general, our team of qualified pharmacists and experts are ready to offer support and assist with queries. Our team speaks 17 different languages and is ready to help.
everyone.org makes the latest approved medicines available to patients, hospitals and doctors around the world, in a legally compliant way. We source innovative medicines at the best possible prices and ensure safe and efficient delivery. We have successfully delivered to over 88 countries worldwide, and have already supported over 11,000 doctors and patients.
Find out more