Reimbursement information on Spinraza (nusinersen)
Spinal Muscular Atrophy (SMA) is a rare genetic disease that affects movement and muscle strength. Nusinersen (which has the brand name Spinraza) is the first and only approved treatment for children and adults with spinal muscular atrophy (SMA). Nusinersen is not a cure, but it has been shown to improve the physical condition and quality of life of patients.
Currently the only approved medicine for
Spinal Muscular Atrophy
Nusinersen (Spinraza) is an approved treatment that may offer hope for some children and adults with Spinal Muscular Atrophy (SMA). It is approved for use in the USA, EU, Brazil, Japan, Switzerland, Canada, and Australia, among others. Clinical trials show that this medicine can have a positive impact on the lives of children and adults with SMA. Although it is not globally available, patients to whom it is not accessible can import this medicine into their country, safely and legally.
What is nusinersen (Spinraza)?
This medicine is an injection for people with SMA. SMA affects motor neurons and is thought to be caused by changes (mutations) in the chromosomes of DNA. Nusinersen (Spinraza) is expected to counteract the behaviour of this mutated DNA, which should help to maintain motor neurons. Nusinersen (Spinraza) is the first and only approved treatment that works in this way.
Learn more about costs and delivery
Is nusinersen (Spinraza) reimbursed in my country?
Contact us for more information about reimbursement options in your country.
How effective is nusinersen (Spinraza)?
The effectiveness of nusinersen (Spinraza) has been tested in clinical trials. These clinical trials were focused on three patient groups affected in different ways: patients with infantile-onset (Type 1) SMA, patients with later-onset (Type 2 and 3) SMA, and presymptomatic infants likely to develop Type 1 or 2 SMA (diagnosed on the basis of genetic tests).
Study in infantile-onset SMA
This first study included infants who were already showing signs of the disease below the age of 6 months. Clinical studies revealed that infants who were given this medicine showed improvements in motor milestones, which included factors such as: the ability to kick, control the head, sit or deambulate (crawl or move around). These milestones were not achieved by infants who weren’t being treated with this medicine.
Improvement
Spinraza
Untreated
Spinraza
Untreated
For more details refer to the prescribing information at the bottom of the page.
Study in later-onset SMA
Children in this group showed signs of the disease after the age of 6 months (Type 2 or 3). Individuals taking nusinersen (Spinraza) significantly increased their ability to complete certain physical activities (measured with a test called HFMSE). The increase was maintained for the whole duration of the study which was approximately 3 years.
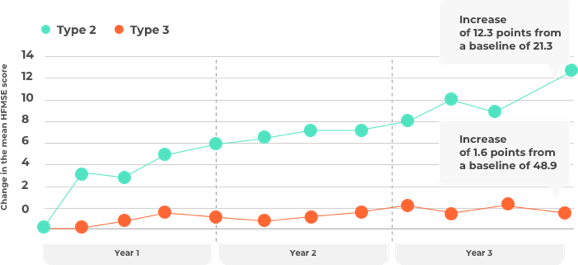
For more details refer to the prescribing information and references at the bottom of the page.
Study in infants with presymptomatic SMA
This ongoing study involved 25 infants who had genetic testing that showed they have SMA (type 1 or 2). They were given the medicine before they had symptoms of SMA.
The latest data show that after over 2 years (25.6 months) the children showed big improvements compared to what is expected for untreated children with SMA type 1 or 2:
- All infants were alive and none required permanent ventilation.
- At the last visit, the average recorded CHOP INTEND* score was 58.4 (out of a maximum score of 64).
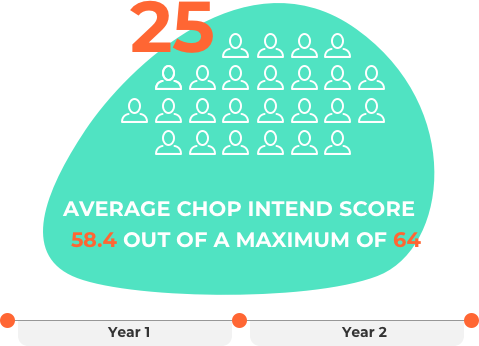
*The CHOP INTEND (Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders) measures general motor function among infants with SMA.
For more details refer to the prescribing information and references at the bottom of the page.
Which side effects can I expect
with nusinersen (Spinraza)?
The most common side effects of nusinersen (Spinraza) include infections to the lower respiratory tract (trachea, bronchi and lungs), fever, reduced or blocked bowel movements, headache, vomiting, back pain, and post-lumbar puncture syndrome (a syndrome that results in leakage of spinal fluid).
Medicines similar to nusinersen (Spinraza) have been shown to result in serious reactions when administered; such as bleeding complications and damage to the kidneys, which can be acute or even fatal.
Please note this is not intended to be a comprehensive guide. Consult your treating doctor and the prescribing information at the bottom of the page for full details of side effects.
How is nusinersen (Spinraza) taken?



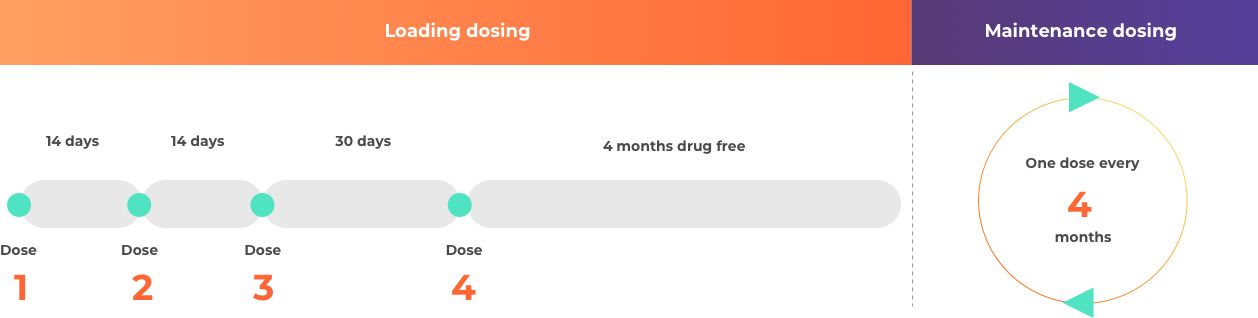
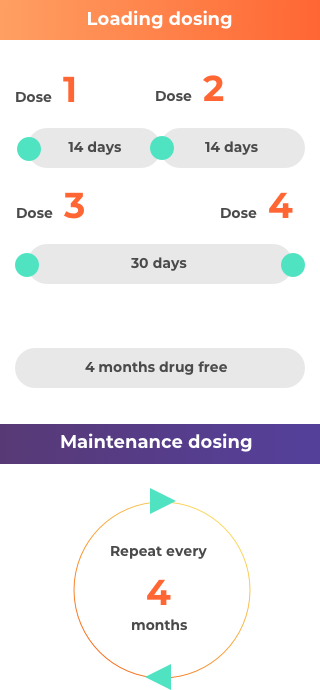
For more details, you can also reference the full prescribing information at the bottom of the page.
How does nusinersen (Spinraza) work?


For more information about the way nusinersen (Spinraza) works, consult your doctor or see the prescribing information at the bottom of the page.
This is not intended to be a comprehensive scientific explanation.

More information about nusinersen (Spinraza)
Need support?
If you are a patient with SMA or have a child with SMA, you might want to ask questions about the medicine for this disease. If you’d like to ask questions about how to get nusinersen (Spinraza), or about our service in general, our team of qualified pharmacists and experts are ready to offer support and assist with queries. Our team speaks 17 different languages and is ready to help.
Who is everyone.org?
everyone.org makes the latest approved medicines available to patients, hospitals and doctors around the world, in a legally compliant way. We source innovative medicines at the best possible prices and ensure safe and efficient delivery. We have successfully delivered to over 88 countries worldwide, and have already supported over 11,000 doctors and patients.
Find out more









