Ocrevus (ocrelizumab) helps preserve hand and arm function in PPMS
Multiple Sclerosis (MS) is an autoimmune disease of the central nervous system, that affects signals sent between the brain and the body. There have been many advances in the treatment of MS in recent years. One of these advances is ocrelizumab (also known under the brand name Ocrevus), which is thought to slow down the progression of the disease.
Currently the only medicine for PPMS
Ocrelizumab (Ocrevus) is an infusion therapy for people with relapsing Multiple Sclerosis (MS) and Primary Progressive Multiple Sclerosis (PPMS). Relapsing Multiple Sclerosis (RMS) is characterised by episodes of worsening function (relapses) which are initially followed by recovery periods (remissions); while Primary Progressive Multiple Sclerosis (PPMS) is characterised by a steady decline from the onset of symptoms, often without early relapses or remissions. It is the first treatment approved for this more aggressive form of the disease.
What is ocrelizumab (Ocrevus)?
Ocrevus (ocrelizumab) is an antibody used for the treatment of people with Relapsing or Primary Progressive forms of Multiple Sclerosis (RMS or PPMS). The patient’s own immune system is supposed to be one of the major causes of the diseases. Ocrelizumab (Ocrevus) works by binding to a specific antigen*, and is thought to hinder the attack on the neurons by the immune system.
Learn more about costs and delivery
* An antigen is a toxin or other foreign substance which induces an immune response in the body.
How effective is ocrelizumab (Ocrevus) for Relapsing Multiple Sclerosis (RMS)?
In two clinical studies, which lasted over two years, ocrelizumab (Ocrevus) was tested on over 1600 patients against another medicine called interferon beta-1a. Ocrelizumab (Ocrevus) reduced the number of relapses experienced by patients compared to interferon beta-1a.
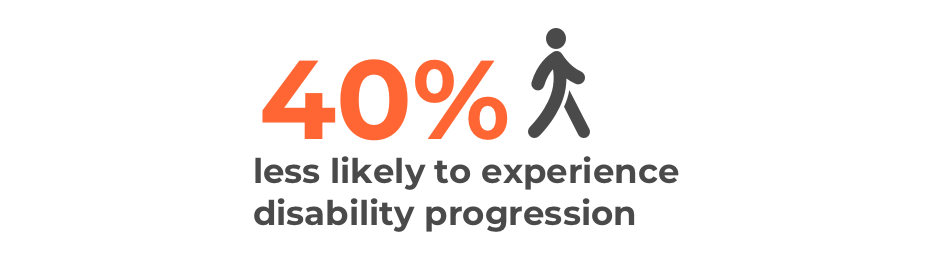
Ocrelizumab (Ocrevus) has been proven to slow disability progression by 40% compared to interferon beta-1a, with 9.8% of people who took ocrelizumab (Ocrevus) experiencing disability progression compared to 15.2% of those who took interferon beta-1a.
Ocrelizumab (Ocrevus) had a significant impact on brain lesions, as evidenced in magnetic resonance imaging scans (MRIs). Specifically, the type of lesions called T1 Gd+ lesions were almost completely suppressed by ocrelizumab (Ocrevus).


For more details refer to the prescribing information and references at the bottom of the page.
How effective is ocrelizumab (Ocrevus) for
Primary Progressive Multiple Sclerosis (PPMS)?
In a clinical study involving a total of 732 patients with PPMS ocrelizumab (Ocrevus) proved its superiority to the placebo in slowing down disability progression. The portion of people having disability progression was 32.9% with ocrelizumab (Ocrevus) and 39.3% with the placebo.
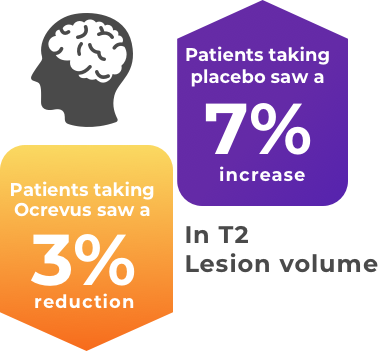
Additionally in patients taking ocrelizumab (Ocrevus) the total portion of the brain affected by brain lesions (T2 hyperintense lesions*) was reduced. The average change in volume of T2 lesions was -0.39 cm3 with ocrelizumab (Ocrevus), representing a reduction in the affected area, compared with an increase of 0.79 cm3 for the placebo.
* T2 hyperintense lesions may reflect new inflammation or older, chronic lesions, which are the scarring left by previous MS activity.
For more details refer to the prescribing information and references at the bottom of the page.
Which side effects can I expect with
ocrelizumab (Ocrevus)?
Some of the most common side effects of ocrelizumab (Ocrevus) are respiratory tract infections, reactions at the site of infusion, skin infections. Some serious adverse reactions include extreme infusion site reactions, increased risk of malignancy (including breast cancer) and infections. Patients should not take ocrelizumab (Ocrevus) if they have a weakened immune system or have had a life-threatening allergic reaction to ocrelizumab (Ocrevus) before.
Please note this is not intended to be a comprehensive guide. Consult your treating doctor and the prescribing information at the bottom of the page for full details of side effects.

You need to keep moving forward. Think what you can do, not what you can't. I'm a lawyer with my own practice and I have been restricted from doing things, but there's always a way.
How is ocrelizumab (Ocrevus) taken?
Ocrelizumab (Ocrevus) is taken as an infusion. First dose: 2 separate infusions 2 weeks apart. Each subsequent dose: 1 infusion every 6 months. Before each infusion, you may be given corticosteroids and an antihistamine to help reduce infusion-related reactions.

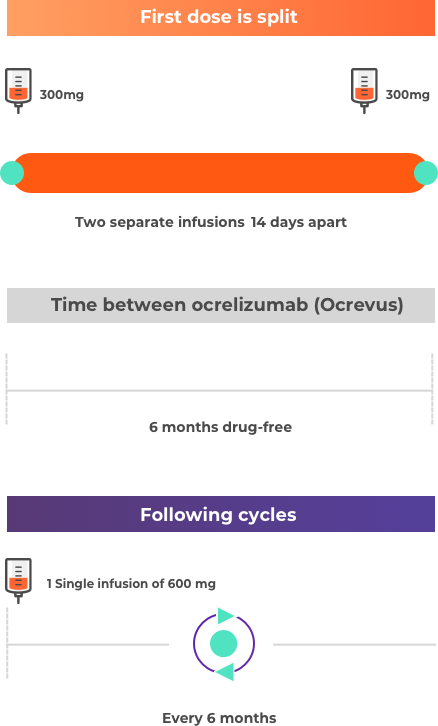
Please note this is not intended to be a treatment plan. For a personalised treatment plan consult your doctor. For more details, you can also reference the full prescribing information at the bottom of the page.
How does ocrelizumab (Ocrevus) work?


For more details about the way ocrelizumab (Ocrevus) works, consult your doctor or see the prescribing information at the bottom of the page. This is not intended to be a comprehensive scientific explanation.

More information about ocrelizumab (Ocrevus)
Need support?
If you are a patient with MS or have a relative with MS, you might want to ask questions about the medicine for this disease. If you’d like to ask questions about how to get ocrelizumab (Ocrevus), or about our service in general, our team of qualified pharmacists and experts are ready to offer support and assist with queries. Our team speaks 17 different languages and is ready to help.
Who is everyone.org?
everyone.org makes the latest approved medicines available to patients, hospitals and doctors around the world, in a legally compliant way. We source innovative medicines at the best possible prices and ensure safe and efficient delivery. We have successfully delivered to over 88 countries worldwide, and have already supported over 11,000 doctors and patients.
Find out more