Ocrevus (ocrelizumab) helps preserve hand and arm function in PPMS
Multiple Sclerosis (MS) is an autoimmune disease of the central nervous system, which affects signals sent between the brain and the body. When multiple sclerosis is highly active cladribine (which is sold under the name Mavenclad), may be an option.
A new low impact option for highly active relapsing MS
Cladribine (Mavenclad) is a medicine for Relapsing Multiple Sclerosis (RMS) for patients whose disease is highly active. Clinical trials have shown that it can provide and maintain up to 4 years of disease control with a maximum of 20 days’ oral treatment and needs little monitoring. The medication is classed as “low impact” because it supposedly has less impact on the day to day lives of patients in comparison to daily medication options. Cladribine (Mavenclad) is approved by the FDA (US) EMA (Europe) and the TGA (Australia). Although it is not globally available, patients to whom it is not accessible can import this medicine into their country, safely and legally.
What is cladribine (Mavenclad)?
Cladribine (Mavenclad) is a medicine used to treat highly active* relapsing multiple sclerosis (RMS). The patient’s own immune system is supposed to be one of the major causes of the disease. The immune cells called lymphocytes play a key role in this process. Cladribine (Mavenclad) is what’s called a nucleoside analog of deoxyadenosine, meaning that it has a similar chemical structure to one of the substances needed to make up the DNA (purine). When taken, cladribine (Mavenclad) leads to the death of the lymphocytes, which in turn, slows down the progression of multiple sclerosis.
The active ingredient, cladribine, is approved in the EU, USA, and Australia, among others, as an intravenous infusion for the treatment of certain leukaemias (cancers affecting lymphocytes).
Learn more about costs and delivery
* Relapsing Multiple Sclerosis (RMS) is defined as highly active when: either two or more relapses have occurred in the previous year OR one or more relapse has occurred while receiving a disease-modifying therapy, along with the appearance of certain brain lesions.
How effective is cladribine (Mavenclad)?
The clinical study involved a total of 1,326 patients with relapsing-remitting MS who had at least one relapse in the previous 12 months. Patients took either cladribine (Mavenclad) or a placebo.
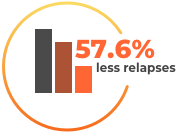


For more details refer to the prescribing information at the bottom of the page.
Which side effects can I expect with
cladribine (Mavenclad)?
Adverse reactions include herpes, lymphopenia (low lymphocyte count), neutropenia (decrease in neutrophil count), rash and alopecia. Lymphopenia (low lymphocyte count) can be serious.
Please note this is not intended to be a comprehensive guide. Consult your treating doctor and the prescribing information for full details of side effects.
How is cladribine (Mavenclad) taken?
The quantity of cladribine (Mavenclad) to be taken depends on the body weight of the patient, and this has an effect on the amount of days per week that they receive treatment.
- Over the course of 2 years: A total of 3.5 mg per kg of body weight, split over 2 years (1.75 mg/kg per year).
- Year 1 and 2: Two treatment weeks, one on the first and one on the fifth week of each treatment year.
- Each treatment week: 4 or 5 days (depending on quantity to be taken) on which a patient receives 1 or 2 tablets as a single daily dose.
- After the 2 treatment years: No further cladribine (Mavenclad) treatment is required in years 3 and 4. Treatment after year 4 has not been studied.
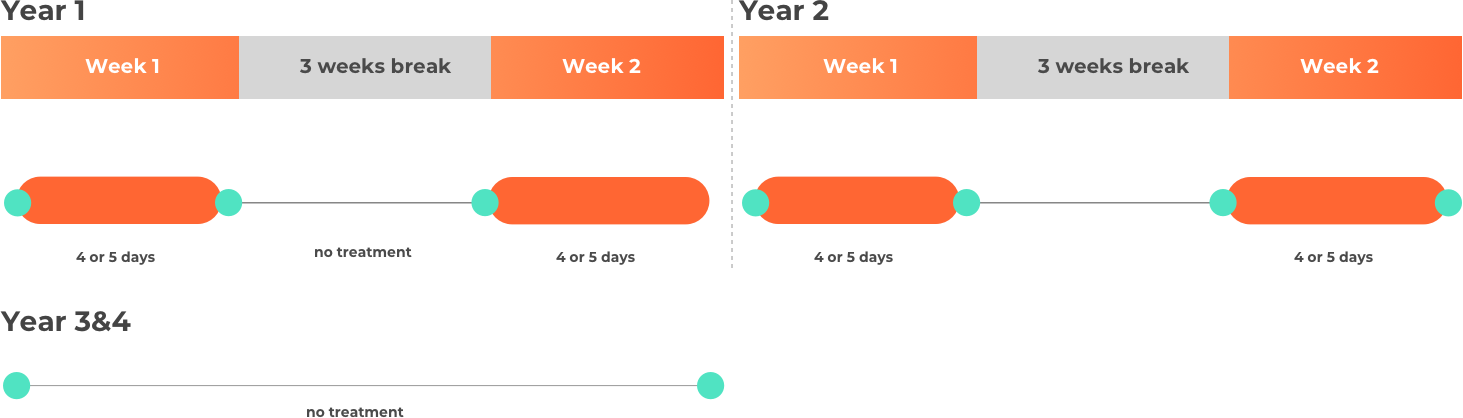
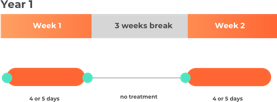
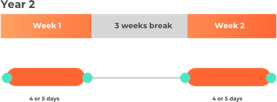
Please note this is not intended to be a treatment plan. For a personalised treatment plan consult your doctor. For more details, you can also reference the full prescribing information at the bottom of the page.
How does cladribine (Mavenclad) work?


For more information about the way cladribine (Mavenclad) works, consult your doctor or see the prescribing information at the bottom of the page. This is not intended to be a comprehensive scientific explanation.

More information about cladribine (Mavenclad)
Need support?
If you are a patient with MS, you might want to ask questions about the medicine for this disease. If you’d like to ask questions about how to get cladribine (Mavenclad), or about our service in general, our team of qualified pharmacists and experts are ready to offer support and assist with queries. Our team speaks 17 different languages and is ready to help.
Who is everyone.org?
everyone.org makes the latest approved medicines available to patients, hospitals and doctors around the world, in a legally compliant way. We source innovative medicines at the best possible prices and ensure safe and efficient delivery. We have successfully delivered to over 88 countries worldwide, and have already supported over 11,000 doctors and patients.
Find out more