Your complete guide to Lumakras (sotorasib) and how to get it
Last updated: 15 January 2024
You can legally access new medicines, even if they are not approved in your country.
Learn howIf you or someone close to you are suffering from non-small cell lung cancer, Lumakras can provide some much-needed hope. Especially in cases where first-line treatments such as chemotherapy or immunotherapy have proven inefficient.
Here, you'll find all you need to know about this promising second-line treatment inhibitor of the KRAS G12-Mutant NSCLC.
What is NSCLC?
There are two forms of lung cancer: small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). Observed under a microscope, NSCLC's cancer cells are larger than those of SCLC. That’s how it got its name.
NSCLC is the most common kind of lung cancer, affecting over 230,000 people each year, in the USA alone. NSCLC tends to progress more slowly than other lung cancers. Around 40% of patients are diagnosed after the cancer has spread to other body parts[2].
What is Lumakras?
Lumakras (sotorasib) is a prescription medicine for advanced non-small cell lung cancer (NSCLC) with KRAS G12C mutation. It's used to treat patients whose tumor has spread and cannot be surgically removed.
Lumakras is a second-line treatment for NSCLC. It's prescribed after the patient has received at least one prior systemic treatment for their cancer[1]. As such, Lumakras is not chemotherapy or immunotherapy. It’s an inhibitor of the KRAS G12C mutated.
In the EU, Lumakras is available under the name Lumykras.
How does Lumakras work?
To understand how Lumakras works, let’s first take a look at what the KRAS G12C mutation in NSCLC is.
What is the KRAS G12C mutation?
KRAS is an oncogene, which means that it can transform cells into cancer cells under specific circumstances. KRAS plays a role in regulating signaling pathways that control the growth, maturation, and death of cells. Mutations in the KRAS oncogene can cause cancer cells in the body to grow and spread more quickly[3].
About 13% of NSCLC patients have a specific KRAS mutation called the KRAS G12C mutation. The same mutation also occurs in 1-3% of colorectal and other tumors[4].
When KRAS genes mutate, the protein they produce remains mostly in an “active” position. This means that it continuously signals to cancer cells to grow.
How Lumakras targets the KRAS G12C mutation
Lumakras is a targeted therapy designed to target only the mutated KRAS G12C molecules and turn them "off". As a result, tumor cell growth may be slowed and prevented [1].
Lumakras is the first targeted therapy of its kind available for NSCLC lung cancer patients with an advanced disease [13].
How effective is Lumakras for NSCLC?
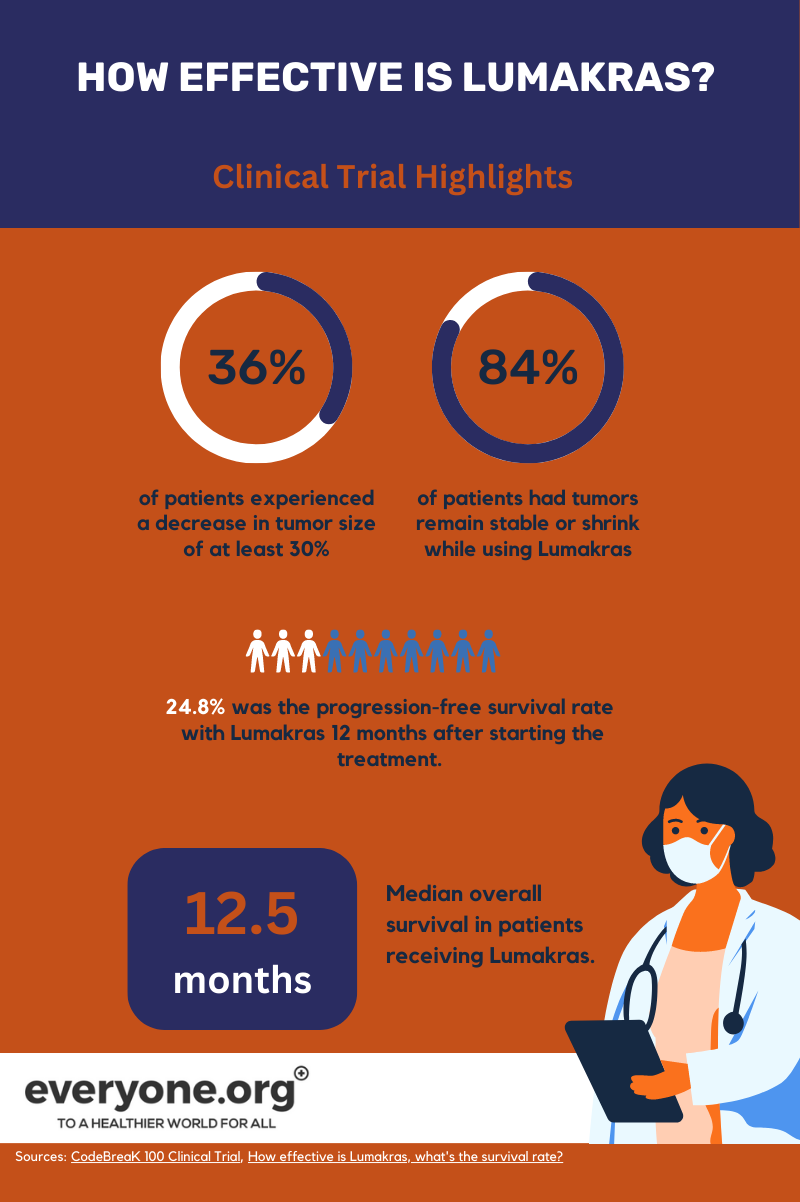
Results from clinical trials of Lumakras revealed that 36% of patients experienced a decrease in tumor sizes of at least 30%. For 58% of these patients, the result lasted for over 6 months[5].
Overall survival rate after 2 years following treatment with Lumakras was found to be 32.5%[6]. This is encouraging, considering that the 5-year survival rate for NSCLC patients with metastases can be as low as 9%[7].

Is Lumakras effective for pancreatic cancer?
There is a growing interest in exploring the potential use of Lumakras as a second-line therapy for other types of cancer beyond NSCLC. Of particular interest are colorectal and pancreatic cancers with KRAS G12C mutation.
Early results from a Phase I/II trial among pancreatic cancer patients with a G12C mutation are encouraging. 84% of patients showed a partial or complete response to the treatment, or their disease remained stable[8].
Despite these encouraging results, Lumakras is not currently approved for the treatment of colon or pancreatic cancer. Before deciding to take Lumakras off-label, it is essential to consult with your doctor to weigh the potential benefits and risks of this approach.
Lumakras (sotorasib) side effects
As with any prescription medication, Lumakras has potential side effects that you should be aware of. Below is a list of the most commonly reported side effects, as well as others that may be more serious and require immediate medical attention. It's always best to talk to your doctor if you are experiencing any symptoms during treatment with sotorasib.
Most common side effects:
- diarrhea
- muscle or bone pain
- nausea
- tiredness
- liver problems
- coughing
- nausea
- abnormal lab tests [1].
Serious side effects:
- The use of Lumakras has been associated with potential breathing complications that could be life-threatening. You should seek medical help immediately if you are experiencing shortness of breath, cough or fever.
- Liver problems. Treatment with sotorasib can result in abnormal liver blood test results. You should be alert for symptoms of potential liver problems, such as your skin turning yellow (jaundice), changes in the color of your stools or urine, nausea, loss of appetite, tiredness or weakness, pain or tenderness on the right side of your stomach[1].


How to take Lumakras
Lumakras is available as a tablet and is administered orally. The recommended daily dose is 960 mg, which is achieved by taking eight 120 mg tablets once daily. You can take the medicine with or without food, at the same time each day[1].
Drugs to avoid while taking Lumakras (sotorasib)
- Antacids. If you take an antacid (such as a Proton Plump Inhibitor or H2 blockers), you should take your Lumakras dose 4 hours before or 10 hours after taking the antacid[1].
- Strong CYP3A4 Inducers. These prescription medicines may reduce the effect of Lumakras. Some of the drugs in this group include: some antibiotics (e.g. rifampin); epilepsy medication (e.g. phenytoin, carbamazepine, phenobarbital); Enzalutamide (a prostate cancer treatment)[9].
- CYP3A4 Substrates, including some corticosteroids and immunosuppresives[10].
- P-gp substrates. Lumakras may reduce the effectiveness of these drugs. Some examples include: medicines preventing organ rejection (e.g. cyclosporin, tacrolimus); some drugs that prevent blood clotting (e.g. rivaroxaban)[9].
Lumakras and alcohol
The interaction between Lumakras and alcohol has not been documented. However, if you are experiencing nausea as a result of the Lumakras treatment, it is recommended that you avoid consuming alcohol[11].
Will I need to take Lumakras long-term?
Second-line therapy with Lumakras is intended to be long-term. Whether this also applies to your case will depend on the clinical benefits and side effects you are experiencing. Your doctor will consult you on the best course of action.
Who is Lumakras (potentially) not suitable for?
- Children. Clinical trials to date have focused on adult patients only. As a result, there is no information about the safety and effectiveness of Lumakras in children[1].
- Pregnant women. As of this point, the potential effects of Lumakras on mother and child during pregnancy have not been studied[1]. It is advised to use birth control during treatment to avoid potential adverse effects on your unborn child[12].
- Breastfeeding women. It's unclear if Sotorasib passes into human milk. Due to the potential for serious side effects in breastfed children, you're advised not to breastfeed during treatment and until 1 week after its end[1].
Where is Lumakras approved?
Lumakras (sotorasib) is currently approved as a second-line treatment for adults with locally advanced or metastatic NSCLC with KRAS G12C mutation in these countries and regions:
- United States
- EU (conditional marketing authorisation under the name Lumykras)
- United Arab Emirates
- Canada (conditional approval)
- United Kingdom (temporary approval)
- Japan.
How to get Lumakras if it's not approved in your country?
You can get access to Lumakras (sotorasib), even if it’s not (yet) approved in your country.
This is possible through the Named Patient Import regulation. It allows for patients with life-threatening or debilitating diseases to purchase medicines not (yet) approved or available in their country, as long as they have been prescribed to them by their doctor.
At Everyone.org, we specialize in accessing, importing and delivering the medicine you need from anywhere in the world to you. We can help you access Lumakras wherever you are.
To find out more and make a request for the medicine, contact us.
References:
1. Full prescribing information [FDA]: Lumakras (sotorasib)[PDF], Amgen, May 2021.
2. Non-Small Cell Lung Cancer > Fact Sheets. Yale Medicine, Accessed May 2023.
3. Definition of KRAS gene - NCI Dictionary of Cancer Terms - NCI. National Cancer Insitute, Accessed May 2023.
4. Targeting KRAS-G12C. Amgen Oncology, Accessed May 2023.
5. CodeBreaK 100 Clinical Trial: LUMAKRAS® (sotorasib). Accessed May 2023.
6. LUMAKRAS® (SOTORASIB) CODEBREAK 100 STUDY SHOWS TWO-YEAR OVERALL SURVIVAL OF 32.5% IN PATIENTS WITH KRAS G12C-MUTATED ADVANCED NON-SMALL CELL LUNG CANCER: Amgen, 10 April 2022.
7. Lung Cancer Survival Rates | 5-Year Survival Rates for Lung Cancer:American Cancer Society, 1 March 2023.
8. LUMAKRAS® (SOTORASIB) SHOWS ENCOURAGING AND CLINICALLY MEANINGFUL ANTICANCER ACTIVITY IN PATIENTS WITH KRAS G12C-MUTATED ADVANCED PANCREATIC CANCER IN CODEBREAK 100 TRIAL. Amgen, 15 February 2022.
9. Lumakras Drug / Medicine Information. News Medical, Accessed 23 May 2023.
10. Table 31.1, [CYP3A4 substrates, inhibitors and inducers...]. - The EBMT HandbookNCBI, Accessed 23 May 2023.
11. Your guide to THE FIRST ONCE-DAILY ORAL TREATMENT1 Not chemotherapy1[PDF]. LUMAKRAS® (sotorasib), Accessed 23 May 2023.
12. Healy, Marisa. Sotorasib (Lumakras™) | OncoLink. Oncolink, 13 July 2021.
13. Lumakras Approved as First Targeted Therapy for Drug Resistant KRAS G12C Lung Cancer Mutation. Global Reach Health, Accessed 23 May 2023.
Disclaimer: This article is not meant to influence or impact the care provided by your treating physician. Please do not make changes to your treatment without first consulting your healthcare provider. This article is not intended to diagnose or treat illness or to influence treatment options. everyone.org is as diligent as possible in compiling and updating the information on this page. However, everyone.org does not guarantee the correctness and completeness of the information provided on this page.





