When will Vamorolone be available in Europe and the UK? The full story.
Last updated: 16 April 2024

You can legally access new medicines, even if they are not approved in your country.
Learn howCorticosteroids have been a standard treatment for patients with Duchenne Muscular Dystrophy for many years. Usually, they are included in the treatment plan around the age of 4 or 5, before entering the "plateau phase" and experiencing a significant loss of strength 1. Unfortunately, long-term use of corticosteroids can be associated with various side effects, including behaviour issues, weight gain, osteoporosis, and more 2.
In this context, the recent FDA approval of Agamree (vamorolone) as a "new-wave corticosteroid" with fewer side effects has been a much-needed breakthrough in Duchenne treatment.
While vamorolone is already expected in US pharmacies in the first quarter of 2024, it hasn't been approved anywhere else yet.
When will vamorolone be available in Europe and the UK? Here's everything you need to know.
What is vamorolone used for?
Agamree (vamorolone) is indicated for the treatment of children (4+) and adults with Duchenne muscular dystrophy.
Agamree is a dissociative corticosteroid. It works mainly by calming down inflammation in the body. Vamorolone acts similarly to other corticosteroids. However, it attaches to cells differently and doesn't activate certain body responses like other steroids do.
It's not completely understood how vamolorone works for DMD (Duchenne Muscular Dystrophy). However, clinical trial results suggest it helps kids with this condition get up faster from lying down and walk better. Some usual side effects include looking swollen, throwing up, gaining weight, and feeling cranky 3.
Is vamorolone approved by the EMA?
Since December 2023, Agamree (vamorolone) has been approved by the EMA for the treatment of Duchenne muscular dystrophy in adults and children over the age of 4 3.
When will vamorolone be available in Europe?
Although vamorolone's EMA approval is good news, it doesn't mean that the medicine will be available right away in all European countries. Before it reaches the pharmacies, the medicine's manufacturer and each member state's local health authority must make decisions about local approval, prices, and health insurance coverage. As a result, vamorolone will likely be available at different times in each country within Europe.
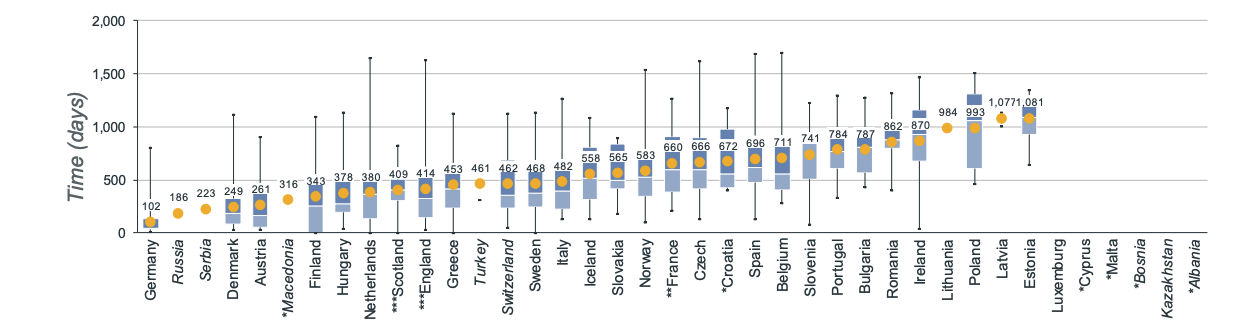
New medicines take on average 511 days after EMA approval to become available across the EU. This timeline is dramatically different per country. For orphan medicines such as vamorolone, It ranges from 102 days in Germany to 1,081 days in Estonia 5.
When will vamorolone be available in the UK?
Following the EMA's approval, vamoroline is now also approved by the MHRA in the UK. This makes the medicine the first Duchenne treatment to be approved in the UK, EU and the US collectively.
Before vamorolone is available on the NHS, NICE has to review and publish its decision on the topic. Consultations are in progress, and a draft guidance is expected by the end of April 2024 6 . If positive, Agamree should be available to patients within 3 months from the NICE's decision date.
Vamorolone approval status in the rest of the world
As of April 2024, Agamree (vamorolone) is not approved anywhere else in the world for the treatment of Duchenne muscular dystrophy 7.

Ways to safely access Agamree (vamorolone) before it's approved in your country
Are you a Duchenne patient outside of the USA? If your doctor thinks vamorolone could help you, you might not have to wait for Agamree's local approval or availability. Instead, your doctor and you could explore vamorolone clinical trials. Or, you can buy vamorolone right away as an Individual Named Patient.
Join a vamorolone clinical trial
You can join a clinical trial to get Agamree (vamorolone) or other unapproved medicines. Finding a trial recruiting participants in your country can be difficult, but it is possible. In order to participate in the trial, you must meet the eligibility criteria. You will also need your treating doctor's support.
Here are some good places to start looking for ongoing vamorolone clinical trials:
- ClinicalTrials.gov: This is a database with all clinical trials in the USA. Some of the trials are also open to international participants. An example is the NCT05185622 clinical trial with vamorolone, which is recruiting for patients in Canada 8.
- EUClinicaltrials.eu: This database contains all clinical trials in the European Union. Currently, it contains limited information on trials launched before 31 January 2022. For those trials, you can refer to the EU Clinical Trials Register.
- myTomorrows: This organization supports patients in finding treatment options in clinical trials.
Buy vamorolone on an Individual Named Patient basis
In most countries, patients are legally allowed to buy and import medicines that could improve their life or address life-threatening conditions. If you want to access vamorolone before it's available in your country, this might be an option for you and your doctor.
The regulation making this possible is known as the Individual Named Patient Import regulation. There may be variations across countries in terms of the specific administrative requirements. However, in all cases these criteria must be met:
-
The medicine in question has market approval in another country and is not (yet) approved or available in the patient's country;
-
There is no alternative on the local market;
-
The medicine is for personal use;
-
The patient has a prescription letter from their treating doctor;
-
The doctor takes responsibility for the treatment. This might require different documentation from country to country.
Do you want to use the Individual Named Patient Import regulation to get vamorolone before it's widely available in Europe, the UK, or elsewhere? You will first need to consult your treating doctor and get a suitable prescription.
Already have a prescription? Our team can support you with buying vamorolone immediately.
References:
- Steroids (corticosteroids). Parent Project Muscular Dystrophy, Accessed 13 November 2023.
- Side Effects of Long Term High-Dose Steroid Therapy, Accessed 13 November 2023.
- Agamree: Authorised | European Medicines Agency. European Medicines Agency, 13 October 2023.
- Applying for EU marketing authorisation for medicinal products for human use. European Medicines Agency, Accessed 13 November 2023.
- EFPIA Patients W.A.I.T. Indicator 2021 Survey. Efpia, Accessed 13 November 2023.
- Project information | Vamorolone for treating Duchenne muscular dystrophy [ID4024] | Guidance. NICE, Accessed 13 November 2023.
- AGAMREE® (vamorolone) – santhera. Santhera, Accessed 13 November 2023.
- A Study to Assess Vamorolone in Boys Ages 2 to <4 Years and 7 to <18 Years With Duchenne Muscular Dystrophy (DMD). ClinicalTrials.gov, Accessed 13 November 2023.




