Tyrvaya: When is the first nasal spray for dry eyes coming to Europe?
Last updated: 06 February 2024
You can legally access new medicines, even if they are not approved in your country.
Learn howDry eye disease is a common, chronic condition that can greatly impact your quality of life. Traditional treatments include artificial tears, gels, ointments, and prescription medicines such as Restasis. In 2021, the FDA approved Tyrvaya, the first nasal spray for dry eyes, thus offering a new approach to managing this condition.
But when will non-US patients be able to benefit from this new treatment?
Here, we'll discuss how Tyrvaya works, its efficiency compared to other medications, and when it will be available in the UK and Europe.
Current Treatment Options for Dry Eye Disease
The management of dry eye disease (DED) traditionally involves a step-by-step approach. Artificial tears are the most widely used first-line therapy. They provide temporary relief by lubricating the eye’s surface. For more persistent cases, doctors may prescribe anti-inflammatory drugs. Examples are cyclosporine eye drops (e.g., Restasis) or lifitegrast (e.g., Xiidra). Both target the underlying inflammation that contributes to DED.
Other treatments include punctal plugs to reduce tear drainage and maintain moisture on the eye's surface. For the most severe cases of DED, surgical intervention may be an option.
The Potential of Tyrvaya Nasal Spray as a Treatment Option
Tyrvaya (varenicline solution) nasal spray represents a new class of treatment for DED. It works differently from eye drops, as it uses the body's neural pathway for controlling tear film production.
Tyrvaya stimulates the trigeminal nerve in the nose to prompt the eyes to produce natural tears. In this way, it aims to restore the natural tear film's stability. This method presents a novel option for those who have difficulty with traditional eye drops. It provides an opportunity to self-administer the treatment via a non-ocular route. This can be especially beneficial for patients with reduced mobility or those who aren't responding well enough to eye drops.
Does Tyrvaya work for Sjogren's?
Sjögren's disease is an autoimmune disorder that primarily affects the moisture-producing glands in the body. The disease is commonly characterized by dryness in the eyes and mouth.
Tyrvaya is currently not approved for use in patients with Sjogren's disease. However, some studies have reported a beneficial effect on patients with the disease. For example, these were the outcomes of a recent study involving 18 patients:
- Tyrvaya showed a trend towards reducing OSDI scores (dry eye symptoms).
- There were statistically significant improvements in dry eye symptoms when using a computer and in windy conditions.
- No significant differences were observed in visual acuity, intraocular pressure, lissamine green staining, or fluorescein staining.
- There was a significant increase in tear production after treatment with Tyrvaya nasal spray 1.
Further data are needed to evaluate the applicability of Tyrvaya to cases of Sjogren's disease, as well as any potential adverse reactions involved.
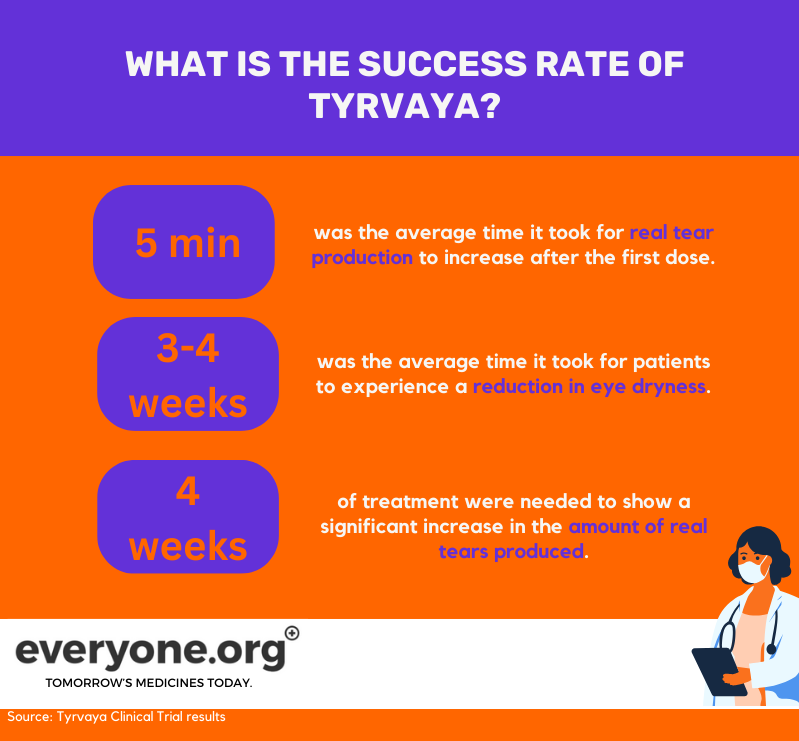
What is the success rate of Tyrvaya?
Tyrvaya's safety and efficacy have been tested in two clinical trials. The key reported results from these trials were:
-
On average, Tyrvaya increased real tear production within 5 minutes after the first dose;
-
It took 3 to 4 weeks of treatment for patients to report a reduction in eye dryness;
-
After 4 weeks of treatment, Tyrvaya showed a significant increase in the amount of real tears produced 2

Is Tyrvaya better than Restasis?
Restasis is an eye drop medicine commonly used to treat dry eye disease. It's difficult to compare Restasis and Tyrvaya, as they have different modes of action and administration.
One potential advantage of Tyrvaya over Restasis is its nasal administration. It could be an advantage for patients with mobility restrictions or those who are not responding well enough to eye drops.
Another point of differentiation between Tyrvaya and Restasis is how quickly they start working. Both medicines seem to offer an initial increase in tear production within 5 minutes of the first dose. However, Restasis may take longer than Tyrvaya to produce its full effect. According to clinical trials data, patients treated with Tyrvaya experienced a significant increase in the amount of real tears produced within 4 weeks 2. For Restasis patients, this may take up to 3 months 3.
Overall, whether Tyrvaya is considered "better" than Restasis largely depends on your specific needs and conditions. Your doctor is best equipped to determine which of the two treatments suits you better.
Tyrvaya EMA Approval: When is it coming?
As of February 2024, no application for Tyvaya's EMA approval has been submitted by Oyster Point Pharma. Unfortunately, this means that the medicine is unlikely to be available on the European market soon.
Typically, a marketing authorization application takes up to 210 days to approve. After that, approximately 2 months are needed to make the approval official. Before an approved medicine becomes available in each EU member state, country-level processes need to be completed to determine local prices and health insurance coverage.
If an application for Tyrvaya's EMA approval were submitted today, it would take approximately 1.5 years for the medicine to be available in some EU countries.
When will Tyrvaya be available in the UK?
There is currently no active application for Tyrvaya's MHRA approval. With this in mind, it's unlikely that the medicine will be available in the UK within 2024.
How can you get Tyrvaya before its EMA and MHRA approval?
Dry eye disease can have a great impact on a patient's daily life. That's why if your doctor believes that Tyrvaya (varenicline solution) could provide you relief, it could be frustrating to have to wait. Especially with no timeline for EMA or MHRA approval in sight.
The good news is, you don't have to wait. If you have a prescription for Tyrvaya from your doctor, you can immediately buy the medicine for your personal use. This is made possible via the Named Patient Import regulation.
Buying Tyrvaya as an Individual Named Patient is the quickest way to access the medicine in Europe and the UK. However, it also means that you would have to cover the medicine costs yourself.
How much does Tyrvaya cost?
When it comes to medicines that aren't widely available yet, any price is an indication. The final price will depend on your location, the supplier, and any import and shipping fees that might apply.
As an indication, one package containing 2 Tyrvaya nasal sprays currently costs approximately EUR 1,036 4.
How long does a bottle of Tyrvaya last?
Each bottle of Tyrvaya is intended to last for 30 days, based on the recommended usage of one spray in each nostril twice daily. This means that your treatment costs per month would be approximately EUR 518, based on the current indicative price for Tyrvaya.
Do you have a prescription for Tyrvaya and are you eager to start your treatment? Get in touch with us and we will happily support you in buying Tyrvaya without waiting for EMA or MHRA approval.
References:
- Efficacy of OC-01 0.03mg varenicline nasal spray in the treatment of Sjogren's Disease. IOVS, Accessed 6 February 2024.
- Clinical Trial Results. Tyrvaya, Accessed 6 February 2024.
- Restasis Eye Drops: Uses & Side Effects. Cleveland Clinic, Accessed 6 February 2024.
- Buy Tyrvaya (varenicline solution) Online • Price & Costs. Everyone.org, Accessed 6 February 2024.