What types of cancer can tazemetostat treat? The latest research.
Last updated: 05 March 2024

You can legally access new medicines, even if they are not approved in your country.
Learn howTazverik (tazemetostat) was the first treatment developed specifically for epithelioid sarcoma 8. However, recent studies are suggesting that it may have a role to play in treating more conditions than that. Including notoriously hard-to-treat solid cancers.
Only time (and clinical trial results) will tell if Tazverik will be applicable to more indications. In the meantime, here's everything worth knowing about the types of cancer it's being studied as a potential treatment for.
What is tazemetostat?
Tazemetostat is a targeted epigenetic regulator. It focuses on EZH2, a protein involved in cell growth regulation. Tazemetostat works by inhibiting mutated forms of EZH2 commonly seen in tumor cells. The goal is slowing down cancer progression 1.
What is Tazverik approved for?
Currently, Tazverik (tazemetostat) is FDA-approved for the treatment of epithelioid sarcoma and follicular lymphoma (with or without an EZH2 mutation) 2. Tazverik's approval was accelerated. Meaning that it was based on the clinical trial data available at the time. However, further clinical trials may be needed to verify its efficacy and to support its approval.
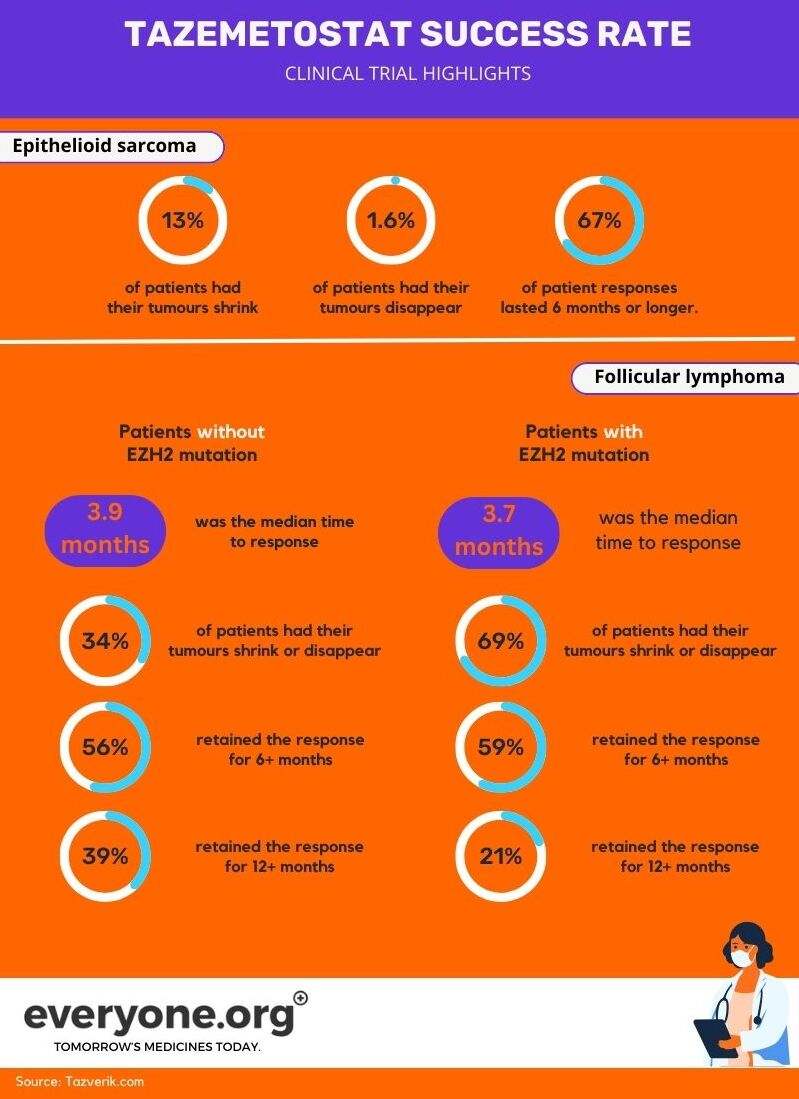
How effective is tazemetostat for epithelioid sarcoma?
The clinical trial which informed the FDA's accelerated approval reported these results:
- 13% of patients treated with tazemetostat experienced tumor shrinkage;
- 1.6% of patients treated with tazemetostat had a complete response (i.e. their tumor completely disappeared);
- 67% of the patients who responded to treatment had this response last 6 months or longer 3.
How effective is tazemetostat for follicular lymphoma?
These are the highlights of the reported clinical trial results:
For patients without EZH2 mutation
- The median time to response was 3.9 months;
- Tumors shrank or disappeared for 34% of patients treated with tazemetostat;
- The median duration of response was 13 months;
- Of those who responded to the treatment 56% retained the response for over 6 months, and 39% - for over 12 months 4.
For patients with the EZH2 mutation
- The median time to response was 3.7 months;
- Tumors shrank or disappeared for 69% of patients treated with tazemetostat;
- The median duration of response was 10.9 months;
- Of those who responded to the treatment 59% retained the response for over 6 months, and 21% - for over 12 months 4.

Can tazemetostat be used for prostate cancer?
Tazemetostat is currently not approved anywhere for the treatment of prostate cancer. However, there is ongoing research and clinical trials exploring its potential role in treating this type of cancer. One example is the ongoing phase 1-2 CELLO-1 study. It explores the combination of Tazverik and Zytiga (abiraterone acetate) or Xtandi (enzalutamide) in patients with metastatic castration-resistant prostate cancer (mCRPC) 5.
These were the key reported results:
- Tazemetostat showed potential in overcoming resistance to androgen-signaling inhibitors (ASI) like abiraterone and enzalutamide.
- Preliminary results showed a decrease in prostate-specific antigen (PSA) levels in some patients, particularly those receiving tazemetostat plus enzalutamide.
- The median radiographic progression-free survival (rPFS) was longer in the enzalutamide group compared to the abiraterone group.
- Safety profiles were generally manageable, with fatigue being the most common adverse event.
The CELLO-1 study is expected to finalize in March 2024. The final results from this and other studies will help us understand better the potential of tazemetostat as a prostate cancer treatment.
Can tazemetostat be used for bladder cancer?
Tazverik is not yet approved for the treatment of bladder cancer. However, early clinical trials have shown some promising results. Specifically, they suggest that tazemetostat, in combination with pembrolizumab, may help slow the growth of bladder cancer by activating the immune system. Further research is still being conducted. However, the initial findings from a pilot study of 12 participants are promising 6:
- 25% of patients had a partial response to the treatment, and 25% had a stable disease.
- The median progression-free survival was 3.1 months;
- The median overall survival was 8.0 months.
The study is ongoing and is expected to finalize in late June 2024. Hopefully, the reported results will provide more insight into the safety and efficacy of tazemetostat and pembrolizumab as bladder cancer treatments.
Can tazemetostat be used for rhabdoid tumors?
Rhabdoid tumors are made up of many large cells. They most often develop in the kidneys and other soft tissues, but they can also grow in the brain. Rhabdoid tumors are aggressive and often affect children. One study with a small number of patients explored how tazemetostat may play a role in rhabdoid tumor treatment 7.
In this small-scale study, 4 pediatric patients were included after completion of chemotherapy. The reported results were:
- 3 out of the 4 patients had an event-free survival of 30 months or over. This was a positive indication, as nearly all relapses occur within two years of diagnosis, which was a point exceeded by the patients in the study;
- The treatment was generally well-torelated. Most patients experienced grade 1-2 nausea or vomiting and no dose reduction was needed as a result. All patients had respiratory and gastrointestinal infections. One patient experienced grade 2 bromide accumulation, which resulted in dose reduction.
More research is needed to better understand the potential role of tazemetostat in treating rhabdoid tumors.
What else can tazemetostat treat?
Tazemetostat is showing potential in a variety of treatment areas. Most are related to tumours, but there is also research indicating that Tazverik may play a role in treating type 1 diabetes.
More data are needed to provide evidence to the efficacy and safety of tazemetostat for any additional indication. However, one thing is certain - the medicine is of high interest for treating doctors and researchers alike. Hopefully, it will also get its EMA approval soon.
Is Tazverik(tazemetostat) not approved or available in your country? If your doctor is of the opinion that this treatment might potentially benefit you, get in touch with our team of medicine access experts. We can give you personalized support for buying Tazverik right now.
References:
- Tazemetostat: EZH2 Inhibitor - PMC. NCBI, Accessed 5 March 2024.
- Reference ID: 4627347. Accessdata.fda.gov, Accessed 5 March 2024.
- HCP ES | Efficacy Data | TAZVERIK. Tazverik.com, Accessed 5 March 2024.
- HCP FL | Efficacy | Clinical Trial Results | TAZVERIK. Tazverik.com, Accessed 5 March 2024.
- Tazemetostat continues to show promise in mCRPC. Urology Times, 1 December 2022.
- A pilot study of tazemetostat and pembrolizumab in advanced urothelial carcinoma (ETCTN 10183), Journal of Clinical Oncology, 21 February 2023.
- Tazemetostat in the therapy of pediatric INI1-negative malignant rhabdoid tumors, Scientific Reports, 07 December 2023.
- FDA approves first treatment option specifically for patients with epithelioid sarcoma, a rare soft tissue cancer. FDA, 23 January 2020.





