Roflumilast Cream in Europe: Approval timelines, efficacy and more
Last updated: 06 February 2024

You can legally access new medicines, even if they are not approved in your country.
Learn howRoflumilast cream, commercially sold as Zoryve, is a topical cream for the treatment of plaque psoriasis and seborrheic dermatitis 1.
Zoryve has been available on the US market since 2022. However, patients in Europe and the UK may be facing a longer wait before they can start a treatment with roflumilast.
Let's take a closer look at Roflumilast cream, what it does, and when it's likely to be available in Europe and the UK.
What is Zoryve (roflumilast) used to treat?
Zoryve, featuring the active ingredient roflumilast, is a topical cream designed to address the symptoms of plaque psoriasis in adults and children over the age of 6. In November 2023, Zoryve also received the FDA's green light for treating seborrheic dermatitis in patients aged 9 and up 1.
Zoryve is a powerful inhibitor of the intracellular enzyme phosphodiesterase-4 (PDE4), which plays a crucial role in causing inflammation associated with plaque psoriasis. By blocking PDE4, Zoryve reduces the production of pro-inflammatory substances.
Zoryve's approval is a step forward in offering patients with plaque psoriasis and seborrheic dermatitis a nonsteroidal, once-daily topical treatment. Zoryve is a viable option for long-term management of dermatological conditions. It effectively addresses inflammation without the negative side effects of prolonged steroid use.
Is Zoryve approved for atopic dermatitis?
Not yet. However, it seems like a matter of time. Zoryve's manufacturer has submitted an application for approval to the FDA. A decision on the application is expected in July 2024 3. If positive, Zoryve cream should be available to US patients with atopic dermatitis shortly after.
Is Zoryve cream a steroid?
Zoryve cream is not a steroid. Its active ingredient roflumilast is a PDE4 inhibitor. It provides an alternative solution for managing plaque psoriasis and seborrheic dermatitis. This avoids the side effects associated with long-term corticosteroid use, making Zoryve a valuable option for patients and prescribers looking for a novel, steroid-free, and effective topical treatment.
How quickly does Zoryve work?
Topical roflumilast starts working immediately. However, it can take up to 2 weeks for it to improve the severity and impact of itching, and up to 8 weeks to experience a significant relief of symptoms. This applies to both psoriasis and seborrheic dermatitis patients.
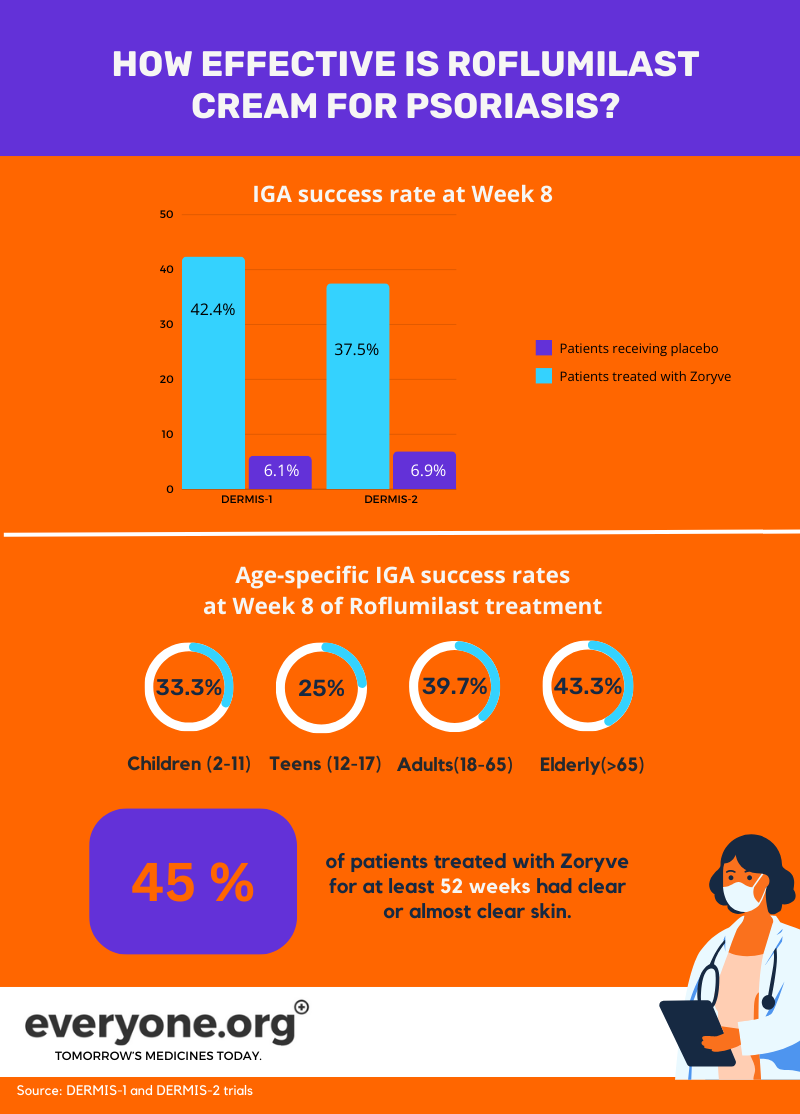
The pivotal trials DERMIS-1 and DERMIS-2 reported that 37-42% of psoriasis patients reached Investigator Global Assessment (IGA) Success by Week 8 of treatment with roflumilast cream. IGA success is defined as a score of clear (0) or almost clear (1) on the IGA score. For patients with seborrheic dermatitis, the rate of reaching IGA success was almost 80% by Week 8 3.
Does roflumilast really work for psoriasis?
This type of questions should always be answered with caution, as results may vary across patients. We can only attempt an answer based on the available clinical trial data.
Efficacy and Safety of Topical Roflumilast in Treating Plaque Psoriasis
In the DERMIS-1 and DERMIS-2 trials, Zoryve was tested against a placebo cream on patients with plaque psoriasis. These were the most important outcomes:
- In trial 1, 42.4% of patients achieved IGA success at week 8, compared to 6.1% of control group patients.
-
In trial 2, 37.5% of patients achieved IGA success at week 8, compared to 6.9% of control group patients.
-
Roflumilast significantly improved itch severity from as early as week 2.
-
It also showed effectiveness in treating intertriginous psoriasis (where skin touches skin).
-
Age-specific IGA success rates at week 8 with roflumilast treatment:
-
Pediatric patients (2-11 years): One-third achieved IGA success.
-
Teenagers (12-17 years): 25% achieved IGA success.
-
Adults (18-65 years): 39.7% achieved IGA success.
-
Elderly patients (older than 65 years): 43.3% achieved IGA success 4.
-
In a long-term safety trial, 45% of patients who continued using Zoryve for at least 52 weeks had clear or almost clear skin 3. These results highlight Zoryve's efficacy and safety in managing psoriasis symptoms.

Future of Roflumilast in Europe: Is approval on the horizon?
While the cream formulation of roflumilast has received FDA approval in the United States, its approval in Europe is not yet a fact.
When will roflumilast cream be available in Europe and the UK?
To become available to patients in Europe and the UK, topical roflumilast needs to get EMA and MHRA approval first. As of February 2024, there is no indication of this happening soon.
Therefore, an exact availability date cannot be provided at this time. Once a marketing authorization application is submitted to the EMA and MHRA, a decision takes up to 210 days. Upon a positive outcome, it can take another few months for Zoryve to become available on the markets in Europe and the UK.
Non-US patients in need of new nonsteroidal, once-daily topical treatments for chronic dermatological conditions may need to wait before being able to start a treatment with roflumilast.
How can you get roflumilast cream before its approval in Europe and the UK?
If your treatment can't wait, it's important to know that you can access roflumilast cream prior to its official approval in Europe and the UK. One way to do so is to try and join a roflumilast clinical trial. Another way is to buy Zoryve cream right away for your personal use.
Here are some details on either option.
Join a Roflumilast Clinical Trial
One of the most straightforward ways to access roflumilast cream before its EMA and MHRA approval is through a clinical trial. If you and your doctor follow this path, you should be aware of the possibility of being assigned to the control group in the trial.
Below are some resources to help you find a suitable trial:
- ClinicalTrials.gov: This is a database with all clinical trials in the USA. However, some of the trials are also open to international participants. At the moment, there are no listed clinical trials involving topical roflumilast, but this can change at any point, so it's worth keeping an eye on.
- EUClinicaltrials.eu: This database contains all clinical trials in the European Union. Currently, it contains limited information on trials launched before 31 January 2022. For those trials, you can refer to the EU Clinical Trials Register.
- myTomorrows and FindMeCure: These organizations support patients in finding treatment options in clinical trials.
Buy Roflumilast Cream as an Individual Named Patient
Most countries in the world allow for patients to import medicines for serious or chronic conditions before they are available locally. The regulations guiding this process can vary in name, but they are typically referred to as Named Patient Import regulations.
If you want to buy Zoryve before its EMA or MHRA approval, this could be a good option for you and your doctor.
To make use of the Named Patient Import regulation, you will need a prescription from your doctor. Based on your country, additional documentation may be required. However, you don't have to stress about it.
If you have a prescription for Zoryve (roflumilast) and would like to buy the medicine right now, get in touch with us. We're qualified to support you with navigating the guidelines and getting the medicine before it’s locally available.
References:
- Stewart, Judith. Zoryve (roflumilast) FDA Approval History. Drugs.com, 2 January 2024.
- HIGHLIGHTS OF PRESCRIBING INFORMATION. Arcutis Biotherapeutics, Accessed 6 February 2024.
- Zoryve (roflumilast) cream and foam. CenterWatch, Accessed 6 February 2024.
- Effect of Roflumilast Cream vs Vehicle Cream on Chronic Plaque Psoriasis: The DERMIS-1 and DERMIS-2 Randomized Clinical Trials. JAMA, 20 September 2022.