Changing the story: How nitisinone brings hope back for HT-1 and AKU patients
Last updated: 15 January 2024

You can legally access new medicines, even if they are not approved in your country.
Learn howWhen faced with a rare disease, finding encouragement can feel as important as finding a treatment. We're here to support you with both, starting with the example of Type 1 hereditary tyrosinemia (HT-1) and Alkaptonuria (AKU).
Even though HT-1 and AKU affect about 1 in 100,000 people, treatments such as nitisinone have helped change the prognosis for patients. And with Early Access Programs (especially in India, Pakistan, Bangladesh, and Sudan), more patients can access treatment, regardless of their financial status.
Here's everything you need to know about Nitisinone and how you can access it.
What Is HT-1?
Hereditary tyrosinemia type 1 (HT-1) is a rare genetic disorder affecting the metabolism of an amino acid called tyrosine. Tyrosine is present in most proteins.
Patients with HT-1 have tyrosine waste buildup in the body. Eventually, this can damage the liver and kidneys, and increase the risk of liver cancer. Some patients can also experience softening and weakening of the bones, as well as problems with their nervous system [1, 2].
How rare is HT-1?
HT-1 affects about 1 in 100,000 people worldwide [3]. Interestingly, in some regions in Canada, the incidence is considerably higher, affecting 1 in 1846 newborns [4]. This is assumed to be due to a founder mutation in the French-Canadian population. A higher incidence of HT-1 is also observed in Turkey and India [4].
Research among different populations in the UK has suggested that people of Pakistani descent may be affected by HT-1 more often compared to those of European descent (3.7 HT-1 cases per million vs 0.04 cases per million, respectively) [5].
Tyrosinemia Symptoms
Symptoms of HT-1 can appear at different points in life. Some patients show symptoms already in their first year, whereas for others they may follow years later.
The types of symptoms differ across patients, and can include rickets, abnormal increases in the spleen or liver size, or acute liver failure [2].
Early symptoms of HT-1 can be difficult to detect. However, starting treatment as early as possible makes a big difference for patient outcomes. That's why newborn screening is important [4].
How Does Nitisinone Treat HT-1?
Nitisinone facilitates the breakdown of tyrosine. This helps to prevent liver and kidney toxicity, along with the damage they cause [7].
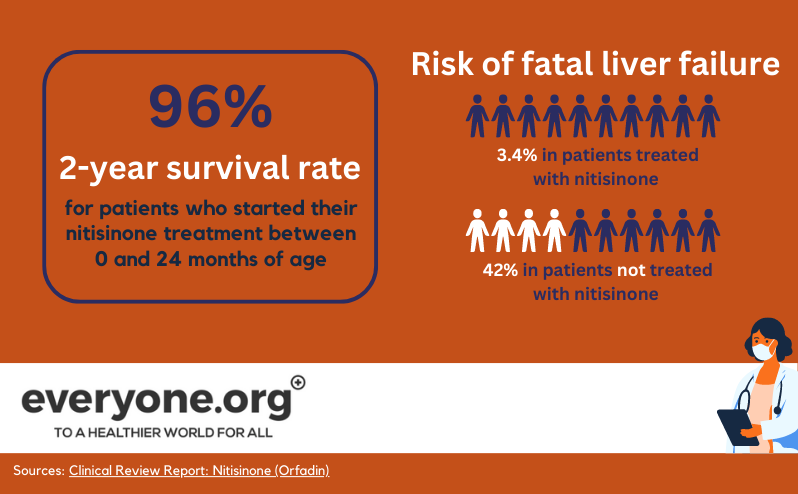
Before the introduction of nitisinone, HT-1 patients would often not live to be teenagers. Liver or kidney failure resulting from toxicity would be fatal for many [6]. Liver transplantation used to be the only way to correct the tyrosine metabolism of HT-1 patients.
Nitisinone tablets have changed this. Over 90% of patients respond very well to treatment with nitisinone, in combination with a low-protein diet. As a result of nitisinone treatment, liver transplants are currently only needed in rare cases [1]. Patients who have started treatment early, live to adulthood and have fairly normal lives [15].

Nitisinone as a treatment for Alkaptonuria (AKU)
Alkaptonuria (also known as Black Bones disease, AKU, or Black Urine disease) is a rare hereditary disease that bears some similarity to HT-1. AKU patients experience disruptions in the metabolism of the homogentisic acid oxidase (HGA) - an enzyme that participates in the metabolism of some amino acids such as tyrosine and phenylalanine[11].
Because both share a similar metabolic pathway, the adequacy of nitisinone as an Alkaptonuria treatment has also been studied [12].
According to study results, nitisinone appears to be an effective treatment for AKU in adult patients, and an effective prevention for AKU complications in children [12]. Also in AKU patients, nitisinone treatment should be combined with dietary restriction of protein, particularly tyrosine and phenylalanine [1].
Further studies are needed in order to confirm nitisinone's efficacy for treating alkaptonuria, as well as establish any potential safety risks for young patients [12].
Types of Nitisinone: Orfadin and Nityr
Nitisinone is available as a generic medication. It's sold under two brand names: Orfadin and Nityr. Orfadin was the first version of nitisinone available on the market. Nityr is bioequivalent to Orfadin. That means, both medicines contain the same active substance (nitisinone) and work in the same way [8].
This being said, there are some differences between the two types of nitisinone treatments.
Orfadin vs Nityr: What is the difference?
While both Orfadin and Nityr work in the same way and are prescribed to treat the same condition (HT-1), they differ from each other in these ways:
- Size. Nityr tablets are much smaller than Orfadin capsules (about 20% of their size) [9]. This can make a difference for patients who have difficulties swallowing.
- Storage. Orfadin capsules need to be refrigerated (or, if stored at room temperature, they need to be used within 45 days or discarded). Nityr tablets don't require refrigeration [10]. This can make them easier to take on the go.
- Approval. Only Orfadin is approved for the treatment of AKU. This approval is only in the EU [16]. However, Orfadin and Nityr are bioequivalent drugs, sharing the same active substance and mechanism of action. That makes it only a matter of time for Nityr to officially get the same approval for the treatment of AKU.
Cost of Nitisinone treatment
How much your nitisinone treatment will cost depends on several factors, including your location, the medicine supplier, and any insurance coverage you may be eligible for.
Whether you are using the medicine for its approved use (HT-1) or off-label use (AKU) will also make a difference. Some insurance companies may not provide coverage for off-label use of a medicine.
As an indication, these are the approximate prices of nitisinone tablets:
- The estimated price of Orfadin for a month's treatment (based on administration twice daily) can vary from around EUR 5,120 to EUR 51,116, depending on the strength required [12].
- For a month's treatment with Nityr, your costs are estimated at around EUR 5,000 to EUR 25,129, depending on the strength required [13].
Nitisinone available for free in India, Pakistan, Bangladesh and Sudan
The manufacturer of Nityr, Cycle Pharma, is currently providing Nityr tablets free of charge for AKU and HT-1 patients in India, Pakistan, Bangladesh and Sudan. This is possible thanks to a partnership established between Everyone.org and Cycle Pharma in 2022.
Please note that some conditions may apply. If you're based in India, Pakistan, Bangladesh or Sudan, please submit a request for Nityr via the page below to find out whether you are eligible for free nitisinone tablets (Nityr).
NOTE: Unfortunately, we are currently unable to deliver to Sudan. However, we can make Nityr available for pick-up in one of our partner pharmacies in Luxembourg, Germany, or the Netherlands. Contact us for more information.
The future of rare disease treatments
Although currently only 5% of rare diseases have a treatment, we see this medical landscape changing every day. Nitisinone is just one example. We can’t wait to share more with you.
References:
- Tyrosinemia Symptoms & Treatment. UPMC Children's Hospital of Pittsburgh, Accessed 2 August 2023.
- Tyrosinemia type 1 - About the Disease. Genetic and Rare Diseases Information Center, Accessed 2 August 2023.
- Feillet, Francois. Treatment Adherence in Type 1 Hereditary Tyrosinaemia (HT1): A Mixed-Method Investigation into the Beliefs, Attitudes and Behaviour of Adolescent Patients, Their Families and Their Health-Care Team. NCBI, 12 September 2014.
- Braekeleer, M., and J. Larochelle. Genetic epidemiology of hereditary tyrosinemia in Quebec and in Saguenay-Lac-St-Jean. NCBI, Accessed 2 August 2023.
- A comparison of disease and gene frequencies of inborn errors of metabolism among different ethnic groups in the West Midlands, UK. Journal of Medical Genetics, May 1998.
-
Das, Martin. Clinical utility of nitisinone for the treatment of hereditary tyrosinemia type-1 (HT-1). NCBI, 24 July 2017.
-
Nitisinone Oral: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing. WebMD, Accessed 2 August 2023.
-
Nityr. European Medicines Agency, Accessed 2 August 2023.
- What is Nityr?. Nityr, Accessed 2 August 2023.
- Reference ID: 4130114. Accessdata.fda.gov, Accessed 2 August 2023.
- Adequacy of nitisinone for the management of alkaptonuria. NCBI, Accessed 2 August 2023.
- Orfadin Prices, Coupons, Copay & Patient Assistance. Drugs.com, Accessed 2 August 2023.
- Nityr Prices, Coupons, Copay & Patient Assistance. Drugs.com, Accessed 2 August 2023.
- Tyrosinemia type I. Myriad Genetics, Accessed 2 August 2023.
- Success in DevelopAKUre: approval for Orfadin to treat patients with AKU. Research and innovation, 26 October 2020, Accessed 2 August 2023.





