Jemperli vs Keytruda for endometrial cancer: What’s the difference?
Last updated: 15 January 2024
You can legally access new medicines, even if they are not approved in your country.
Learn howIf you've been exploring treatments for dMMR/MSI-H endometrial cancer, you've likely come across chemotherapy as the standard. But the recent FDA approvals of Jemperli (dostarlimab) and Keytruda (pembrolizumab) are changing things.
Both immunotherapy medicines have received wide media attention. And while the media sometimes calls them "miracle drugs", it's essential to cut through the noise and stay with the facts 12.
If you're looking for a quick and simple way to understand how Jemperli is different from Keytruda, you're in the right place.
In this article, we'll compare the two medicines in the context of endometrial cancer.
Jemperli vs Keytruda: What are they used for?
Similarities
Both Jemperli (dostarlimab) and Keytruda(pembrolizumab) are prescribed as second-line treatments for advanced endometrial cancer that is microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR).
Differences
- Only Jemperli can be used as a first-line treatment as well. It's prescribed alongside chemotherapy (carboplatin and paclitaxel) for adult patients with primary advanced dMMR/MSI-H endometrial cancer.
- Only Keytruda is approved to treat advanced mismatch repair proficient (pMMR) endometrial cancer. In this capacity, Keytruda is used as a second-line treatment, in combination with chemotherapy (lenvatinib).
Dostarlimab vs Pembrolizumab: How do they work?
Both medicines are immunotherapies and their active ingredients - dostarlimab and pembrolizumab - work in a similar way.
When T cells (a type of immune cell) come into contact with certain substances (PD-L1 and PD-L2), they can get "switched off" and stop fighting against threats, such as tumors. Some tumors can increase the presence of PD-L1 and PD-L2 to turn T cells off, so they can grow undisturbed.
Dostarlimab and pembrolizumab aim to prevent this by sticking to the "off switch" (PD-1 receptor) on the T cells. This helps keep the immune system active against the tumor 5,6.
How efficient are Jemperli and Keytruda for endometrial cancer?
Keytruda clinical trial results
-
Advanced MSI-H/dMMR endometrial cancer
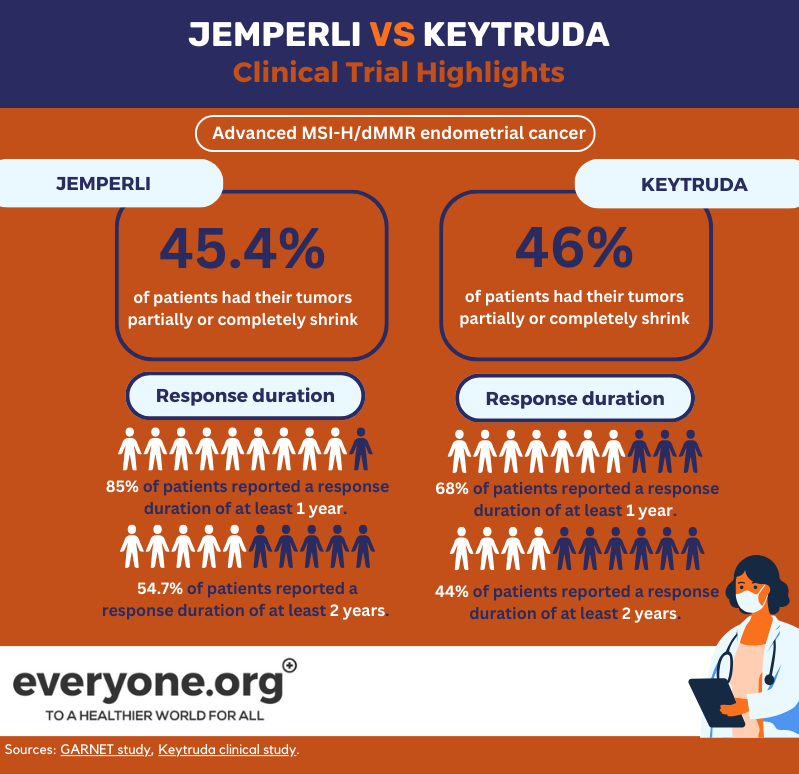
Keytruda's safety and efficacy in advanced MSI-H/dMMR endometrial cancer was tested in a clinical trial. Participants received 200 mg of Keytruda once every 3 weeks.
The results of the clinical trial reported that 46% of people had their tumors partially or completely shrink. The treatment response lasted for at least a year in 68% of patients, and in 44% of patients - for at least 2 years 2.
- Advanced pMMR endometrial cancer
According to the published results of a phase 3 trial, patients treated with Keytruda(pebrulizumab) in combination with chemotherapy had a median progression-free survival rate of 13.1 months, as compared to 8.7 months when treated with placebo in combination with chemotherapy 1.
Jemperli clinical trial results
Jemperli has been tested in two clinical trials (RUBY and GARNET).
The RUBY trial tested Jemperli plus chemotherapy against placebo with chemotherapy in treating primary advanced or recurrent dMMR/MSI-H endometrial cancer.
In the RUBY trial, Jemperli and chemotherapy showed, according to the reports, a 71% reduction in the risk of disease progression or death, as compared to placebo and chemotherapy. Patients treated with Jemperli and chemo reported a median progression-free survival (PFS) of 30.3 months, compared to 7.7 months for patients treated with placebo and chemo.
Overall, 73.8% of patients treated with Jemperli and chemo responded to the treatment (47.6% partially, and 26.2% completely). For 61.3% of patients, this effect was still seen and reported after over a year 3.
The GARNET study focused on testing Jemperli as a stand-alone second-line treatment for advanced or recurrent dMMR/MSI-H endometrial cancer.
In the GARNET trial reports, 45.4% of patients responded to the Jemperli treatment (29.8% partially and 15.6% completely). 85.9% of responding patients still had the same effect after a year. 54.7% - after 2 years 4.

Jemperli vs Keytruda: Safety and side effects
According to their prescribing information, these are the most common side effects of Jemperli (dostarlimab) and Keytruda(pembrolizumab):
Jemperli side effects
When used in combination with carboplatin and paclitaxel (in patients with primary advanced or recurrent dMMR/MSI-H endometrial cancer):
- rash;
- diarrhea;
- hypothyroidism;
- hypertension (high blood pressure) 6.
Based on the published trial results, fatal adverse reactions occurred in 6% of patients receiving Jemperli in combination with chemotherapy. Serious adverse reactions occurred in 13% of patients 6.
When used as a single agent (in patients with recurrent or advanced dMMR endometrial cancer):
- tiredness;
- anemia;
- diarrhea;
- nausea 6.
Fatal adverse reactions occurred in 0.7% of patients treated with Jemperli alone, and serious adverse reactions - in 38% of patients 6.
Keytruda side effects
When used in combination with lenvatinib (in patients with advanced pMMR endometrial cancer):
- hypothyroidism;
- hypertension (high blood pressure);
- tiredness;
- diarrhea;
- musculosceletal disorders;
- nausea;
- decreased appetite;
- stomatitis;
- weight loss;
- stomach pain;
- urinary tract infection;
- proteinuria;
- constipation;
- headache;
- hemorrhagic events;
- palmar-plantar erythrodysesthesia;
- dysphonia;
- rash;
- hepatoxicity;
- acute kidney injury 5.
During Keytruda's clinical trial, fatal adverse reactions occurred in 4.7% of patients treated with Keytruda and chemotherapy. Serious adverse reactions occurred in 50% of patients 5.
When used as a single agent (in patients with advanced dMMR/MSI-H endometrial cancer):
- tiredness;
- musculoskeletal pain;
- rash;
- diarrhea;
- pyrexia;
- cough;
- decreased appetite;
- pruritus;
- dyspnea;
- constipation;
- pain, including stomach pain;
- nausea;
- hypothyroidism 5.
Based on these data, it seems that Jemperli may have a more favorable safety profile compared to Keytruda, when it comes to treatment of endometrial cancer.
Jemperli vs Keytruda: Price comparison
When it comes to medicines that aren't widely available yet, it's difficult to indicate an exact price. Final costs tend to vary depending on your location, suppliers, or insurance coverage. The prices shown below are indicative only.
How much does Jemperli cost?
To give you an idea, a single-dose vial of Jemperli (500 mg) costs approximately EUR 6,798. A year's treatment would require approximately 16 vials (6x 500 mg every 3 weeks, followed by 5 x 1000 mg every 6 weeks). Based on this calculation, the yearly Jemperli treatment costs would be around EUR 108,768 per patient.
How much does Keytruda cost?
When it comes to Keytruda, a 100 mg vial of the medicine costs about EUR 4,226. The recommended dosage for endometrial cancer patients is 200 mg every 3 weeks or 400 mg every 6 weeks. Depending on the administration plan your doctor recommends, the yearly treatment costs with Keytruda would be in the range of EUR 120,863 - EUR 145,374 per patient.
Where are Jemperli and Keytruda approved for endometrial cancer treatment?
Jemperli(dostarlimab) is approved by the FDA in the USA and the EMA in Europe 10, 11.
Keytruda (pembrolizumab) has broader approval, including in the USA, EU, Canada, Australia, and New Zealand 7,8,9.
Note that the approvals mentioned here only refer to the medicines' use in the treatment of endometrial cancer. Both Jemperli and Keytruda have approvals for treating other types of cancer as well. These may differ per country.
Is Jemperli or Keytruda not (yet) approved or available in your country? If you and your doctor are of the opinion that these treatments might potentially benefit you, get in touch with our team of medicine access experts. We can give you personalized support and source the medicine for you.
References:
- Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. The New England Journal of Medicine, June 8 2023.
- Clinical Trial Results With Advanced MSI-H/dMMR Endometrial Cancer. Keytruda.com, Accessed 4 September 2023.
- RUBY trial results. Jemperli, Accessed 4 September 2023.
- GARNET Efficacy & Study Design. Jemperli, Accessed 4 September 2023.
- Highlights of prescribing information [PDF]. Keytruda, Accessed 4 September 2023.
- JEMPERLI (dostarlimab-gxly) injection. GSKPro for Healthcare Professionals, Accessed 4 September 2023.
- Merck, Eisai's Keytruda-Lenvima combo wins simultaneous OKs in U.S., Canada and Australia. Fierce Pharma, Accessed 4 September 2023.
- Keytruda: Pending EC decision | European Medicines Agency. European Medicines Agency, 21 July 2023.
- FDA approves pembrolizumab for advanced endometrial carcinoma. FDA, 21 March 2022.
- Jemperli | European Medicines Agency. European Medicines Agency, 21 April 2021.
- FDA Approves Immunotherapy for Endometrial Cancer with Specific Biomarker. FDA, 22 April 2021.
- Dostarlimab as a Miracle Drug: Rising Hope against Cancer Treatment. NCBI, 8 August 2022.