How effective is Radicava/Radicut (edaravone) for ALS?
Last updated: 01 November 2019

You can legally access new medicines, even if they are not approved in your country.
Learn howAn easy-to-understand infographic displaying the results from the clinical trial
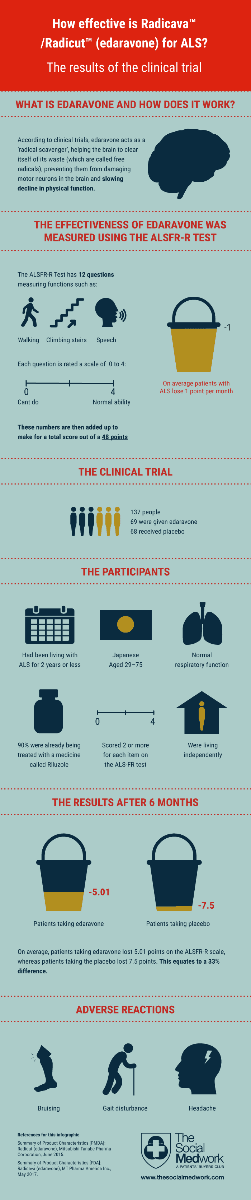
If you're trying to decide whether or not to try the newlyFDA approved medicine Radicava (edaravone) to treat amyotrophic lateral sclerosis (ALS), chances are that one of the first things you looked at were the results from the clinical trial.
However if you're not from a science background it can be tricky to understand all the medical terminology and statistics, so we thought we'd create an infographic summarising the clinical trial results in an easy-to-read format.
We hope it's helpful – feel free to Tweet, Pin and share it with others who may also find it a useful resource! (You're most welcome to republish this infographic on your own website etc., as long as you don't make any changes to it and credit everyone.org as the creator.)
Where is edaravone available?
Outside of the USA and Japan, edaravone is not approved and therefore may not be available as there is no global, harmonised approval system. It is possible in most countries, however, to import medicines on a ‘named patient’ basis. If you or a loved-one is looking for an ALS medicine that is not yet available in your country, read our home page to understand how our team can help. Our team delivers not yet approved medicines from around the world on daily basis, with service that’s highly rated by doctors and patients.
*Side note: 'Radicut' and 'Radicava' are simply different country brand names for the exact same active ingredient, edaravone, produced by the original manufacturer, Mitsubishi Tanabe Pharmaceuticals. More information about that in an earlier blog that we wrote.




