Can Enhertu treat colorectal cancer? Latest results, approval timelines, and more.
Last updated: 15 July 2024
You can legally access new medicines, even if they are not approved in your country.
Learn howSince its first FDA approval in 2019, Enhertu has gained a lot of attention in the world of oncology. In 2023, Enhertu continues its impressive trajectory with two additional FDA breakthrough designations. It now boasts a total of seven 1.
One of Enhertu's latest achievements is its approval as a cancer-agnostic treatment. This means that it can now be applicable to all patients with HER2-expressing tumors, including colorectal tumor. For all colorectal cancer patients, this is an important milestone. At the same time, it poses a lot of questions.
Here's all you need to know about Enhertu for colorectal cancer.
Does Enhertu have FDA approval for colorectal cancer?
Yes. In April 2024, Enhertu was approved for use across all cancer types where a HER2-expression is present. This also incudes colorectal cancer.
What types of cancer is Enhertu approved for?
Enhertu is currently approved by the EMA in Europe for the treatment of:
- HER2-positive metastatic breast cancer, which cannot be surgically removed;
- HER2-low metastatic breast cancer, which cannot be removed by surgery;
- HER2-positive advanced gastric cancer (stomach cancer) or gastro-oesophageal junction cancer 4.
In the USA, Enhertu is additionally approved for the treatment of:
- HER2-mutant metastatic Non-Small Cell Lung Cancer 5.
- all HER2-expressing solid tumors.
How effective is Enhertu for colorectal cancer?
Since Enhertu is a targeted therapy, it's only applicable to cancers with a HER-2 expression. About 3-5% of all colorectal cancers fall under this category 6.
Clinical trial results
In June 2023, the final results of the DESTINY-CRC01 trial were published. This phase 2 trial focused on assessing the efficacy and safety of Enhertu in patients with HER2-expressing metastatic colorectal cancer. The patients in the trial had tumors that progressed after at least 2 prior rounds of treatment 7.
The key outcomes of this trial for colorectal cancer patients were:
-
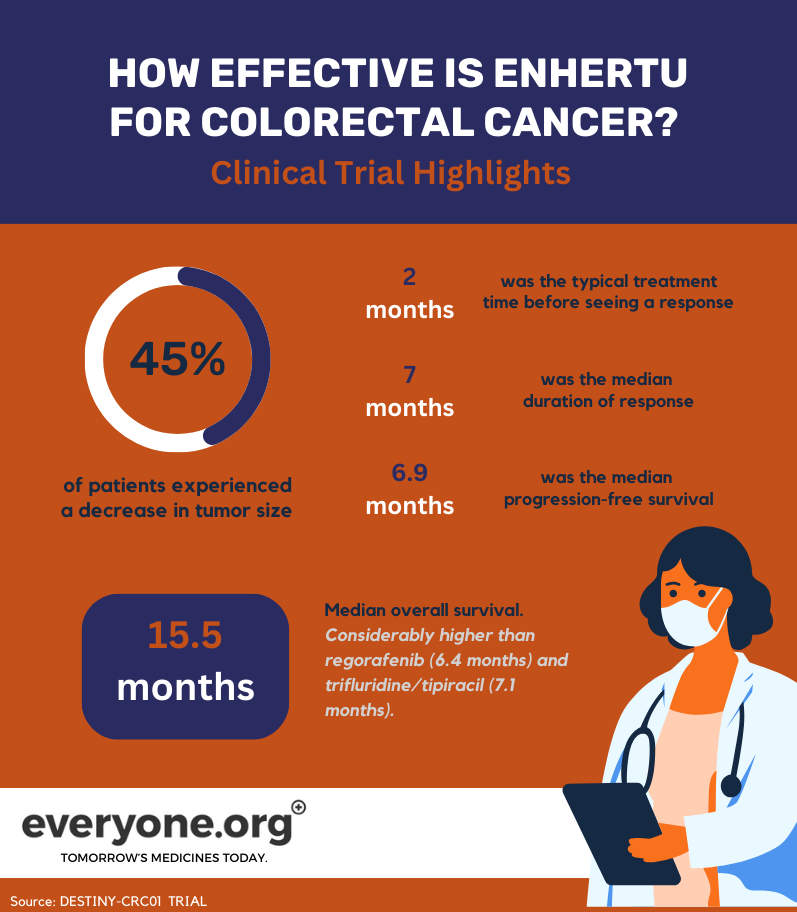
45.3% of patients treated with Enhertu experienced partial shrinkage to their tumors;
-
The response to treatment was typically seen about 2 months after treatment start;
-
The median duration of response was 7 months;
-
The median progression-free survival was 6.9 months;
-
The median overall survival was 15.5 months. This result exceeds significantly the current standard of care. For instance, regorafenib and trifluridine/tipiracil are common third-line therapies. These treatments have an overall survival of 6.4 months and 7.1 months, respectively 7.
Another trial, called DESTINY-CTC02, is studying Enhertu for colorectal cancer patients. The trial is still ongoing, but its preliminary results are in line with the outcomes of the DESTINY-CTC01 trial 10.

How safe is Enhertu for colorectal cancer?
In terms of safety, these are the main results from the DESTINY-CRC01 trial:
-
Each patient had a side effect from the treatment. The most common side effects were stomach and blood problems.
-
Serious adverse effects occurred in 37.7% of patients. The most common serious side effects were low neutrophil counts (22.1%) and anemia (14%) 7.
-
A side effect called interstitial lung disease (ILD) was associated with discontinuation of the drug and was observed in 7% of patients.
-
3 drug-related deaths were reported, all of which were associated with interstitial lung disease.
What does this mean?
Enhertu's safety results cannot be directly compared between different patient groups. However, to help you put things in context, here is a summary of Enhertu's safety results across multiple tumor types. Including tumors for which Enhertu is approved:
-
ILD occurred in 12% of trial patients, with fatal outcome in 1.5% of cases;
-
Decreased neutrophil counts occurred in 34.6% of trial patients;
-
Anaemia occurred in 34-43% of trial patients (depending on medication dosage) 8.
Considering this, the safety profile of Enhertu for colorectal cancer is similar to its profile for other approved cancers.
When will Enhertu be approved for colorectal cancer?
Enhertu is already approved by the FDA for HER2-positive colorectal cancer - an indication that falls within the latest FDA approval of Enhertu.
However, this latest approval is only a fact in the USA. What does this mean for colorectal cancer patients elsewhere? What role could Enhertu play in your CRC treatment?
Can my doctor prescribe Enhertu for colorectal cancer?
The short answer is yes.
Enhertu is already FDA-approved for HER2-positive colorectal cancer treatment. Even if you're based in another country, your doctor has the authority to prescribe the medicine for this indication anyway. They could do this based on the final results of the DESTINY-CRC01 trial or the preliminary results of the DESTINY-CRC02 trial, and on the specifics of your case.
If a doctor gives you a medicine for a disease it's not locally approved for, that's off-label use. In some countries, filling an off-label use prescription may be challenging due to local regulations and availability. However, it's always possible to fill it using the Named Patient Import regulation.
Has your doctor made the decision to prescribe Enhertu for the treatment of your colorectal cancer? Our team at www.everyone.org can help you access the medicine. We specialize in sourcing and delivery of prescribed medicines unapproved or unavailable in a patient's country. Contact us, so we can help you.
References:
- Barrie, Robert. Enhertu wins two FDA breakthrough therapy designations. Pharmaceutical Technology, 31 August 2023.
- FDA Grants Breakthrough Therapy Designations to Trastuzumab Deruxtecan for HER2+ Solid Tumors, Including mCRC. OncLive, 31 August 2023.
- Breakthrough Therapy. FDA, 4 January 2018.
- Enhertu | European Medicines Agency. European Medicines Agency, Accessed 30 October 2023.
- Stewart, Judith. Enhertu (fam-trastuzumab deruxtecan-nxki) FDA Approval History. Drugs.com, 15 August 2022.
- HER2 Biomarker in Colon Cancer. Know Your Biomarker, 24 May 2023.
- Final results of DESTINY-CRC01 investigating trastuzumab deruxtecan in patients with HER2-expressing metastatic colorectal cancer. Nature Communications, Accessed 30 October 2023.
- Enhertu, INN-trastuzumab deruxtecan. European Medicines Agency , Accessed 30 October 2023.
- Trastuzumab Deruxtecan in Participants With HER2-overexpressing Advanced or Metastatic Colorectal Cancer (DESTINY-CRC02). ClinicalTrials.gov, Accessed 30 October 2023.
- Trastuzumab deruxtecan (T-DXd) in patients (pts) with HER2-overexpressing/amplified (HER2+) metastatic colorectal cancer (mCRC): Primary results from the multicenter, randomized, phase 2 DESTINY-CRC02 study. Journal of Clinical Oncology, Accessed 30 October 2023.