Your complete guide to Briumvi, the newest approved MS treatment
Last updated: 15 January 2024
You can legally access new medicines, even if they are not approved in your country.
Learn howMultiple Sclerosis (MS) is a complex and debilitating neurological disease that affects millions of people worldwide. While there is currently no cure for MS, there are various MS treatment options available. They can help manage the symptoms and slow down the progression of the disease.
One emerging treatment that has gained attention is Briumvi (ublituximab-xiiy). But what exactly is Briumvi and how does it work in the context of MS?
In this article, we will delve into the details of Briumvi and its potential benefits for individuals living with MS.
What is Briumvi?
Briumvi (ublituximab-xiiy) is a prescription medicine used to treat adult patients with relapsing multiple sclerosis (RMS). This includes clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease.
Briumvi isn't suitable for patients infected with the hepatitis B virus (HBV)[1].
How does Briumvi work?
To understand how Briumvi works, let's first have a look at how MS affects the brain.
The role of B cells in MS
Multiple sclerosis is a disease in which the immune system is heavily involved. In healthy people, the immune system helps fight off diseases. However, in patients with MS, it mistakenly attacks the central nervous system, destroying the myelin of healthy cells. Myelin is part of the protective sheath of nerve fibers [3]. As its destruction and MS progress, the outermost layer of the brain (cerebral cortex) shrinks.
For years, it was thought that T cells within the immune system were mostly responsible for the progression of MS. Recent research evidence supports the idea that B cells also play an important role [2]. B cells can deplete antibodies directed at the protein CD20. This depletion seems to be related to MS relapse and neurological deficiencies[4].
How Briumvi addresses B cells in MS
Briumvi (ublituximab-xiiy) is a monoclonal antibody. It targets B cells which express the protein CD20 and it binds to them. By suppressing these immune cells, Briumvi aims to reduce the frequency and severity of MS relapses, delay disability progression, and decrease the number of active brain lesions seen on MRI scans [4].
What is the difference between Briumvi, Kesimpta and Ocrevus?
Briumvi is the latest, but not the only CD20 antibody to get FDA approval for treating MS. Two more similar medicines were previously approved - Kesimpta (ofatumumab) and Ocrevus (ocrelizumab).
You're probably asking yourself whether Briumvi is better than Ocrevus or Kesimpta. According to key opinion leaders interviewed by GlobalData, all three medicines are quite similar [6].
One of Briumvi's potential advantages over Kesimpta and Ocrevus is the somewhat easier administration. Briumvi infusions are hour-long and only needed twice a year. In comparison, Ocrevus requires two 2-hour infusions per year. Kesimpta infusions are required every four weeks.
What is the success rate of Briumvi?
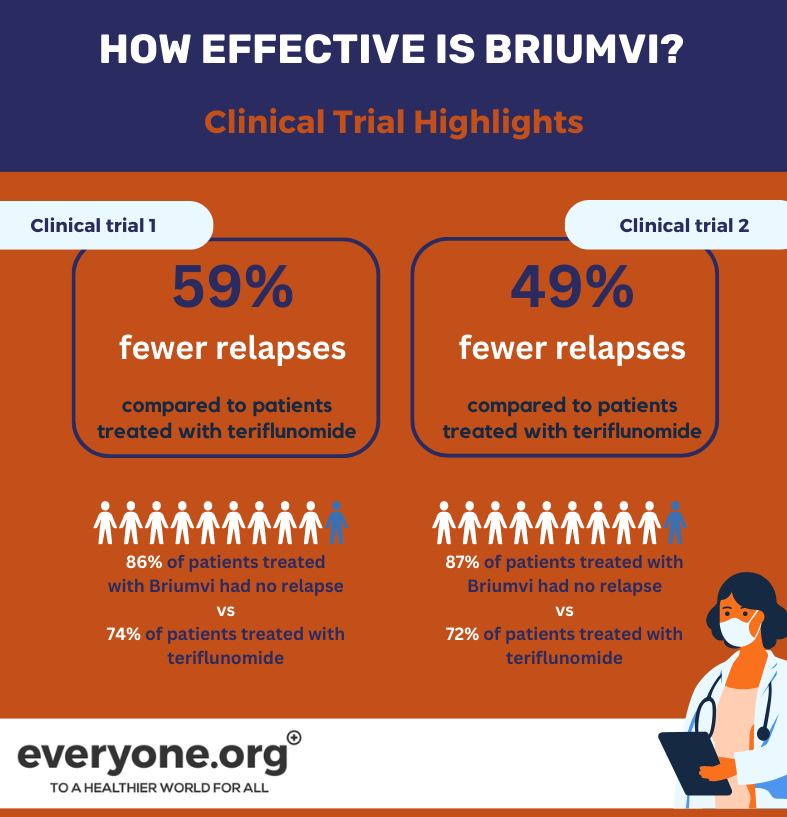
The safety and efficacy of Briumvi has been tested in 2 clinical trials. Both compared Briumvi to another MS treatment (teriflunomide).
Patients treated with Briumvi had 59% (1st clinical trial) and 49% (2nd clinical trial) fewer relapses compared to those treated with teriflunomide [5].
Another finding from the trials was that more people taking Briumvi vs teriflunomide had zero relapses. In trial 1, 86% of Briumvi patients vs 74% of teriflunomide patients had no relapse. In trial 2, these numbers were 87% of Briumvi patients vs 72% of teriflunomide patients [5].

What are the side effects of Briumvi?
Most commonly, Briumvi causes infusion reactions. They can happen even after 24 hours have passed since your infusion. That's why it's important to contact your doctor if you experience any of these symptoms:
- Fever
- Headache
- Flu-like symptoms
- Fast heartbeat
- Hives or itchy skin
- Dizziness
- Swelling of the tongue or throat
- Trouble breathing
- Wheezing
- Nausea
- Abdominal pain
- Throat irritation
- Redness of the face or skin [1].
Other side effects of Briumvi include:
- Infections. Most commonly, of the upper respiratory tract [1].
- Low immunoglobulin levels [1].
Can Briumvi cause PML?
There is no evidence that progressive multifocal leukoencephalopathy (PML) may be caused by a treatment with Briumvi [7].
PML is a viral brain infection. It's caused by the JC virus and usually affects people that are immunocompromised.
There have been cases of patients treated with other anti-CD20 antibodies and MS therapies, who developed a JC virus infection and PML. However, there has been no documented case of PML following a Briumvi treatment [7].
How to take Briumvi
Briumvi is administered as an intravenous infusion. The first infusion lasts about 4 hours. Two weeks later, you will receive a second, 1-hour-long infusion, followed by a 1-hour long infusion every 6 months [1].
Your doctor will likely give you a corticosteroid, an antihistamine, or another medicine before your first Briumvi infusion. This aims to reduce the risk of infusion reactions [1].
Drugs to avoid while taking Briumvi
Some immune-modulating or immunosuppressant medicines may increase the risk of infection, if taken together with Briumvi [7]. To avoid unwanted effects, it's very important to discuss all medicines you're taking with your doctor, before starting your treatment.
Where is Briumvi approved?
In December 2022, Briumvi received the FDA's approval for the treatment of relapsing multiple sclerosis (RMS) in the USA [8].
In the EU, Briumvi received the European Commission's approval for use in the treatment of RMS in June 2023. It's expected that Briumvi will be available to patients in Europe in late 2023 [9].
How much does Briumvi cost?
If a medicine is unapproved or unavailable in your country, it's difficult to get a clear price estimate. The final costs may vary based on your supplier, your location, or insurance coverage.
As a rough indication, a single dose of Briumvi costs around EUR 15,130. Starting your treatment will require an initial infusion, a second infusion within 2 weeks, and an infusion every 6 months after that. This means that the yearly costs for your Briumvi treatment can add up to EUR 45,390 in the first year (3 infusions), and EUR 30, 260 (2 infusions) per year after that [10].
How to get Briumvi if it's not approved in your country?
You can get access to Briumvi (ublituximab-xiiy), even if it’s not (yet) approved or available in your country.
This is possible through the Named Patient Import regulation. It allows for patients with life-threatening or debilitating diseases to purchase medicines not (yet) approved or available in their country, as long as they have been prescribed to them by their doctor.
At Everyone.org, we specialize in accessing, importing and delivering the medicine you need from anywhere in the world to you. We can help you access Briumvi wherever you are.
To find out more and make a request for the medicine, contact us.
References:
- Patient Medical Guide Brochure. Briumvi, 13 June 2023.
- Anti-CD20 therapies in multiple sclerosis: From pathology to the clinic. NCBI, 23 March 2023.
- Multiple Sclerosis | National Institute of Neurological Disorders and Stroke. National Institute of Neurological Disorders and Stroke, 23 January 2023.
- Arneth, Borros M. Impact of B cells to the pathophysiology of multiple sclerosis - Journal of Neuroinflammation. Journal of Neuroinflammation, 25 June 2019.
- Clinical trial results. Briumvi, Accessed 14 July 2023.
- Briumvi may struggle to make headway in crowded multiple sclerosis market. Pharmaceutical Technology, 27 January 2023.
- Briumvi (Ublituximab-xiiy Injection): Uses, Dosage, Side Effects, Interactions, Warning. RxList, Accessed 14 July 2023.
- TG Therapeutics Announces FDA Approval of BRIUMVI™ (ublituximab-xiiy). Investor Relations | TG Therapeutics, Inc., 28 December 2022.
- Wexler, Marisa, and Patricia Inacio. Briumvi approved in Europe for active, relapsing forms of MS –...” Multiple Sclerosis News Today, 2 June 2023.
- Buy Briumvi (ublituximab-xiiy) Online. Everyone.org, Accessed 14 July 2023.