Briumvi vs Ocrevus vs Kesimpta: Understanding Your MS Treatment Options
Last updated: 15 January 2024

You can legally access new medicines, even if they are not approved in your country.
Learn howIf you're navigating the landscape of Multiple Sclerosis (MS) treatments, Briumvi, Ocrevus, and Kesimpta may be on your radar. This overview will help you grasp the key similarities and differences between these three significant MS treatments.
It covers everything from their uses, mechanisms of action, administration methods, efficacy, side effects, to costs and approvals.
Brumvi vs Ocrevus vs Kesimpta: What are they used for?
Similarities
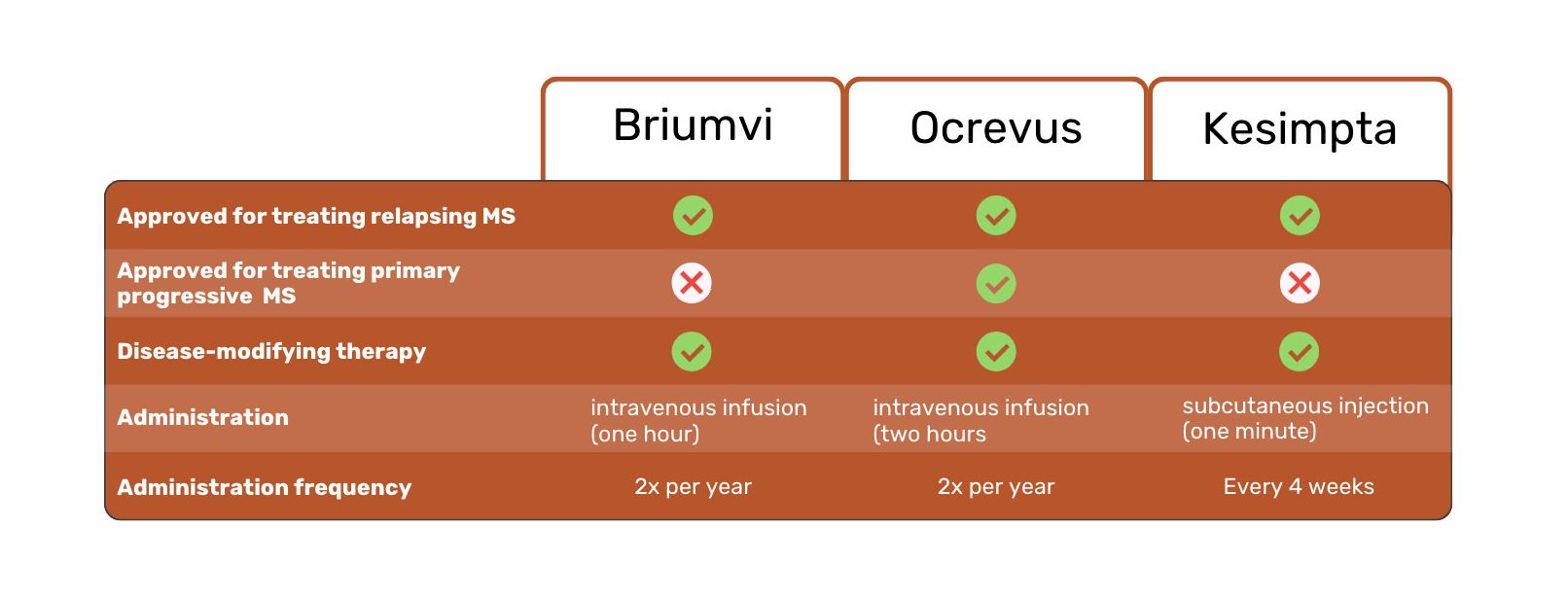
All three therapies are prescribed for the treatment of Multiple Sclerosis (MS). More specifically, relapsing MS (RMS), including clinically isolated syndrome, relapsing-remitting MS, and active secondary progressive MS 1,2,3.
Differences
Ocrevus is the only medicine of the three with FDA approval for treating patients with primary progressive MS (PPMS) 1.
Briumvi vs Ocrevus vs Kesimpta: How do they work?
Briumvi, Ocrevus and Kesimpta are all disease-modifying therapies. In other words, they aim to slow the progression of MS and address its underlying cause, rather than focusing on its symptoms alone.
The three medicines are anti-CD20 monoclonal antibodies and are overall quite similar in their mechanism of action. It is not fully understood how they work, but all three medicines target specific B cells which express the protein CD20. These cells are believed to be involved in the progression of MS.
By attacking these B cells, Briumvi, Kesimpta and Ocrevus aim to reduce MS relapse rates, slow disease progression, and decrease active brain lesions in MS patients 4.
How are Briumvi, Ocrevus and Kesimpta administered?
There are some notable differences in the administration of the three therapies.
Briumvi and Ocrevus are available as intravenous infusions. Kesimpta is injected in the skin instead. This is a major difference, as it allows Kesimpta patients to self-inject at home, instead of receiving an infusion at a hospital or clinic.
Another difference between the three MS treatments is the frequency and duration of administration. Briumvi requires one-hour infusions twice a year. In comparison, Ocrevus requires two 2-hour infusions per year. Kesimpta injections are required every four weeks.

How efficient are Briumvi, Ocrevus and Kesimpta?
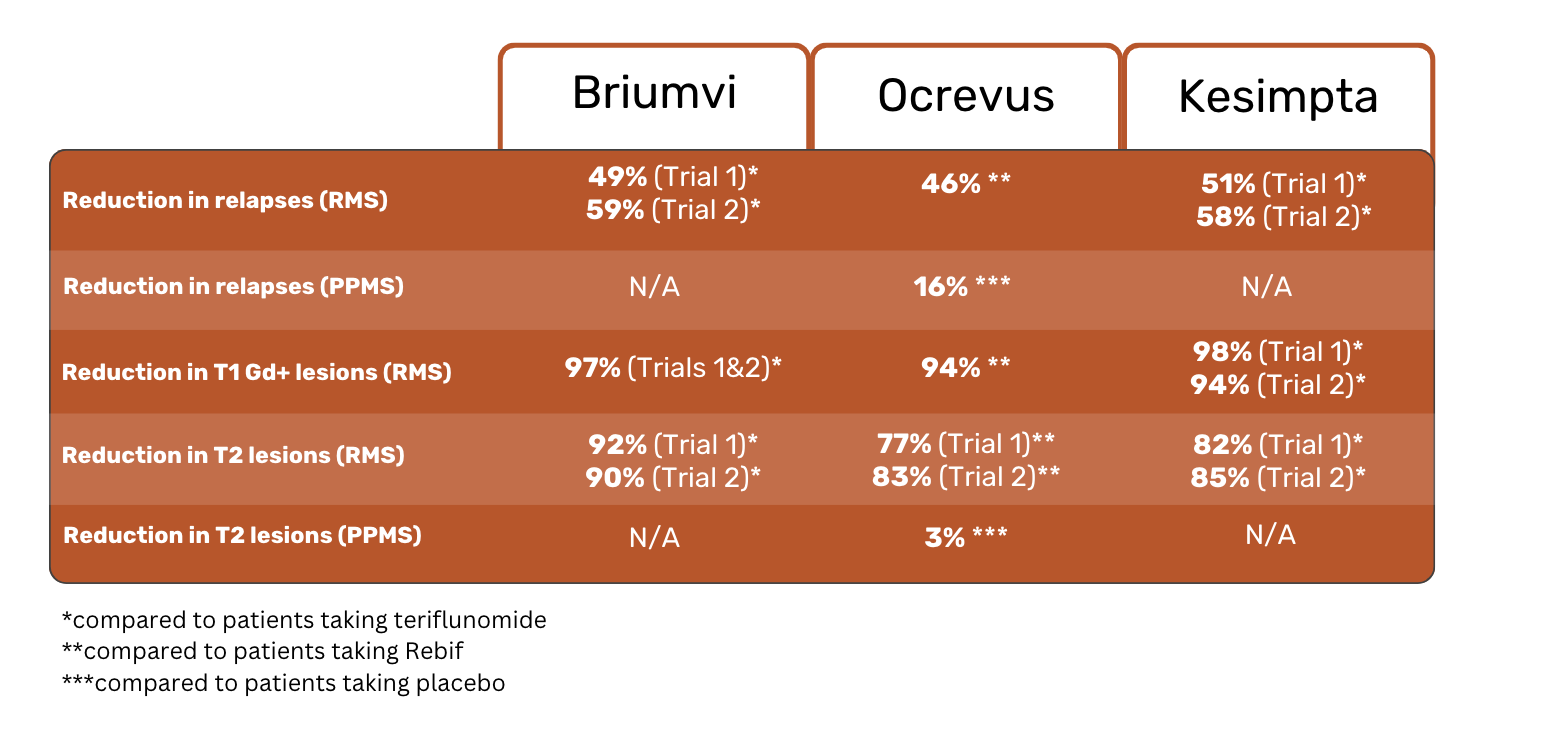
In terms of efficacy, all three medicines have shown similar results in reducing relapses and slowing the progression of RMS. Below are some highlights from their clinical trial results.
Ocrevus clinical trial results
-
Results in RMS patients
In 2 clinical trials spanning over 2 years, Ocrevus was tested against another approved MS treatment, Rebif.
The results of the studies showed a 46% reduction in relapses in Ocrevus patients, as compared to Rebif patients. 83% of those taking Ocrevus experienced no relapses, compared to 71% of those taking Rebif 4.
Over the 2-year span of the studies, people taking Ocrevus were 40% less likely to have disability progression than those taking Rebif. Regarding T1 Gd+ lesions, Ocrevus patients experienced 94% fewer lesions than Rebif patients. Reducing lesions is considered an important element in slowing MS progression 4.
-
Results in PPMS patients
To assess its safety and efficacy in PPMS patients, Ocrevus was tested against a placebo.
Clinical study results showed that 32.9% of patients taking Ocrevus experienced disability progression, as compared to 39.3% of those taking placebo.
Placebo patients experienced a 7% increase in their T2 lesion volume, whereas Ocrevus patients saw a 3% reduction in T2 lesion volume.
Briumvi clinical trial results
Briumvi's efficacy and safety were tested in 2 clinical trials against another MS treatment - teriflunomide.
Clinical trial results showed that Brumvi patients experienced 49% (Trial 1) and 59% (Trial 2) fewer relapses than teriflunomide patients. Of the people taking Briumvi, 86% (Trial 1) and 87% (Trial 2) had no relapses, compared to 74% (Study 1) and 72% (Study 2) of teriflunomide patients.
Briumvi patients had 97% (Trials 1 & 2) fewer T1 Gd+ lesions than teriflunomide patients.
Kesimpta clinical trial results
Similar to Briumvi, Kesimpta was tested against teriflunomide in 2 clinical trials.
Results showed 51% (Trial 1) and 58% (Trial 2) fewer relapses in Kesimpta patients compared to teriflunomide patients.
Disability progression after the onset of disability symptoms was 34% (3 months after symptoms onset) and 33% (6 months after symptoms onset) less likely for Kesimpta patients than for teriflunomide patients.
Kesimpta patients experienced 98% (Trial 1) and 94% (Trial 2) fewer T1 Gd+ lesions compared to teriflunomide patients. Kesimpta patients also had 82% (Trial 1) and 85% (Trial 2) fewer T2 lesions.

Briumvi vs Ocrevus vs Kesimpta: Safety and side effects
According to their prescribing information, the most common side effects associated with Briumvi, Ocrevus and Kesimpta treatments are mostly similar. An overview, including some nuances, is listed below.
Briumvi side effects
- Infusion reactions, including fever, chills, headache, flu-like symptoms 5.
- Infections. The overall rate of infections was 56%, with 5% considered serious. The most commonly reported infections were of the upper respiratory tract and urinary tract 5.
Ocrevus side effects
- Infusion reactions, including itchy skin, rashes, difficulty breathing, swelling and soreness of the throat, low blood pressure, fever, tiredness 6.
- Infections. The overall rate of infections was 58%. The most commonly reported infections were of the upper- and lower respiratory tract, as well as skin infections and herpes-related infections 6.
Kesimpta side effects
- Injection-related reactions, including swelling, itching and pain 7.
- Infections. The overall rate of infections was 51.26%-52.7%, with 1.8%-2.5% considered serious. The most commonly reported infections were of the upper respiratory tract and the urinary tract 7.
- Headache.
Do Briumvi, Kesimpta or Ocrevus lead to progressive multifocal Leukoencephalopathy (PML)?
PML is a rare viral infection of the brain, usually observed in immunocompromised people. Some cases of PML have been reported after treatment with Ocrevus 6.
Even though there are no currently reported cases of PML after a Briumvi or Kesimpta treatment, it's important to keep in mind that PML may happen after treatment with any of these therapies.
Briumvi vs Ocrevus vs Kesimpta: Price comparison
When it comes to medicines that aren't widely available yet, costs tend to vary depending on your location, suppliers, or insurance coverage.
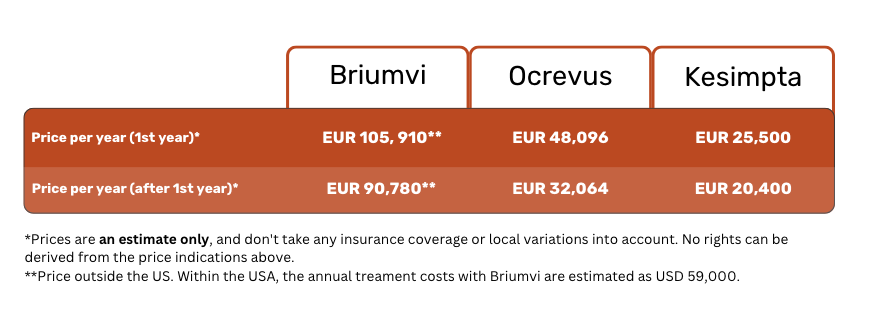
Below is an approximation of yearly treatment costs with Briumvi, Ocrevus, and Kesimpta, assuming no insurance coverage.
How much does Briumvi cost without insurance?
For patients in the USA, where Briumvi is approved and available on the market, the annual treatment cost is estimated as USD 59,000. This sum covers 2 infusions of 450 mg (6 vials in total). At this price, Briumvi is the most affordable MS treatment in the USA at the moment 13.
However, the situation differs for patients in the EU and other regions outside the USA. While Brumvi is approved in the EU, it is not yet commercially available there, resulting in higher sourcing costs. As soon as EU pricing for Briumvi is set and the treatment becomes available locally, the price for non-US patients is likely to also go down. We’ve seen a similar trend with Kesimpta and Ocrevus, which have been on the EU market for several years.
In the meantime, a single dose of 150 mg (6 mL) of Briumvi outside of the USA costs around EUR 15,130. This translates to an annual treatment cost of about EUR 90,780 (6 vials).
How much does Kesimpta cost without insurance?
A single dose of 20 mg (0.4 mL) of Kesimpta costs around EUR 1,700.
Treatment usually starts with an initial dose of 20 mg (0.4 mL), repeated one week after and two weeks after. Subsequent injections are done once a month.
This means that your yearly treatment costs will be about EUR 25,500 in the first year, and go down to about EUR 20,400 afterwards.
How much does Ocrevus cost without insurance?
A single dose of Ocrevus (300 mg/mL) costs around EUR 8,016.
Treatment typically begins with an initial dose of 300 mg, repeated two weeks later. Subsequent infusions are done once every 6 months, at a recommended dosage of 600 mg.
This adds up to about EUR 48,096 of treatment costs in the first year, going down to EUR 32,064 after that.

Where are Briumvi, Ocrevus and Kesimpta approved?
Briumvi and Kesimpta are currently approved only in the USA and the EU 8, 9, 10, 11. Ocrevus is currently approved in over 98 countries, including the USA, the EU, Canada, Australia, New Zealand 12.
Is Briumvi, Ocrevus or Kesimpta not (yet) approved or available in your country? If you and your doctor are of the opinion that these treatments might benefit you, get in touch with our team of medical access experts. We can give you a personalized price quote for sourcing the medicine for you.
References:
- OCREVUS® (ocrelizumab) | Multiple Sclerosis (MS) Treatment. Ocrevus, Accessed 22 August 2023.
- Relapsing MS Treatment I KESIMPTA® (ofatumumab). Kesimpta, Accessed 22 August 2023.
- BRIUMVI ® (ublituximab-xiiy). Briumvi, Accessed 22 August 2023.
- OCREVUS® (ocrelizumab) Results for RMS (Relapsing MS). Ocrevus, Accessed 22 August 2023.
- Reference ID: 5101565. TG Therapeutics, Accessed 22 August 2023.
- HIGHLIGHTS OF PRESCRIBING INFORMATION. Genentech, Accessed 22 August 2023.
- HIGHLIGHTS OF PRESCRIBING INFORMATION. Novartis, Accessed 22 August 2023.
- Briumvi | European Medicines Agency. European Medicines Agency, 13 July 2023.
- Approval of BRIUMVI™ (ublituximab-xiiy) | TG Therapeutics, Inc. TG Therapeutics, 28 December 2022.
- FDA Approves Kesimpta® (Ofatumumab), the First Self-Administered B-cell Therapy for Relapsing Forms of MS | MSAA. Multiple Sclerosis Association of America, 25 August 2020.
- Kesimpta | European Medicines Agency. European Medicines Agency, 29 January 2021.
- Ocrevus. Roche, Accessed 22 August 2023.
- FDA Approves Briumvi (ublituximab-xiiy) for Relapsing MS. National MS Society, 28 December 2022.