Adakveo vs Oxbryta: Getting to know your crizanlizumab alternatives
Last updated: 15 January 2024
You can legally access new medicines, even if they are not approved in your country.
Learn how
Following Adakveo (crizanlizumab)'s revoked authorization in the EU, many patients and doctors need to find an Adakveo alternative.
If you've responded well to your treatment, you can still access Adakveo even if it's not approved in the EU anymore. However, if your doctor prefers to change your treatment course, it's good to understand what this means for you as a patient.
In this article we'll look at one of the possible Adakveo alternatives your doctor may want to discuss - Oxbryta (voxelotor). Here you'll find a quick overview of the similarities and differences between Adakveo and Oxbryta.
Adakveo vs Oxbryta: What are they used for?
Both Adakveo and Oxbryta are prescription medicines indicated for the treatment of sickle cell disease 1,2. Each of the medicines, however, targets a different aspect of the disease.
Adakveo aims to reduce the frequency of painful vaso occlusive crises, whereas Oxbryta is used to treat haemolytic anemia in sickle cell patients 1,2.
What age is Oxbryta approved for in the EU?
Unlike Adakveo, which can only be used in patients aged 16 years and up, Oxbryta is also indicated for pediatric patients.
In the USA, Oxbryta can be prescribed as of the age of 4, whereas in the EU, pediatric use is only approved from the age of 12 1,2.
Crizanlizumab vs Voxelotor: How do they work?
While both medicines aim to reduce complications of sickle cell disease, they target different aspects of the disease.
Adakveo and the reduction of painful crises
Adakveo aims to reduce the frequency of painful crises, which occur when malformed blood cells get stuck in blood vessels.
The medicine's active ingredient, crizanlizumab, is a specific type of protein known as a monoclonal antibody. This protein is formulated to bind to P-selectin, a substance found on the outer layer of cells lining the blood vessels.
P-selectin helps cells stick to the blood vessels and is involved in vessel blockages during painful episodes in sickle cell disease. By connecting to P-selectin and inhibiting its function, Adakveo aims to prevent these painful episodes 3.
Oxbryta and the prevention of anemia
Oxbryta, on the other hand, aims to prevent anemia associated with sickle cell disease.
The active ingredient in Oxbryta, voxelotor, enhances hemoglobin's capacity to retain oxygen and stops it from forming stiff chains within the blood vessels. This helps the red blood cells keep their normal shape and flexibility. It also reduces the rate of their premature destruction and extends their life 4.
How efficient are Adakveo and Oxbryta?
The efficacy of Adakveo (crizanlizumab) and Oxbryta (voxelotor) has been studied in clinical trials.
-
Adakveo clinical trial results
Adakveo's FDA approval, as well as its initial marketing authorization in the EU were based on the results of the SUSTAIN clinical study. The study compared Adakveo (with or without hydoxyurea) against placebo.
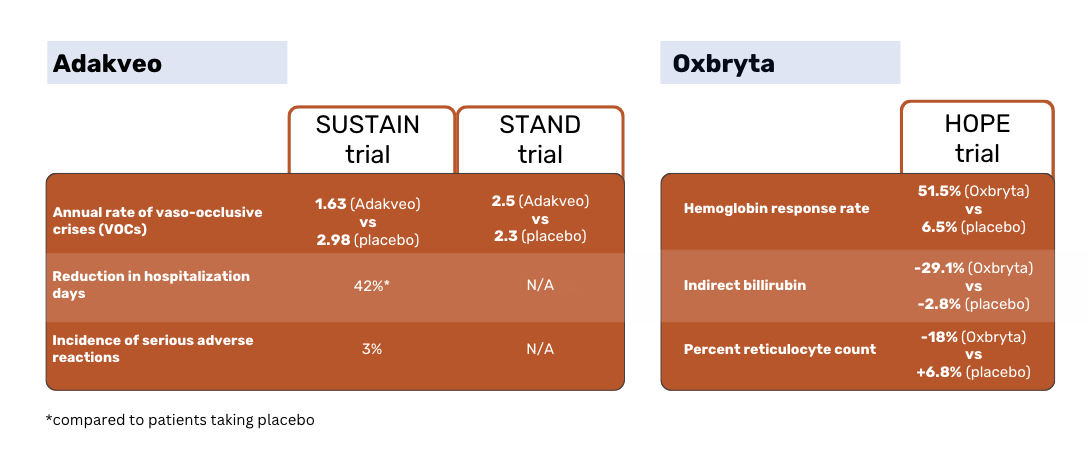
According to the SUSTAIN study results, patients treated with Adakveo had a statistically significant lower median annual rate of vaso-occlusive crises (VOCs) - 1.63 vs 2.98 for placebo patients. 36% of Adakveo patients in the trial had no VOCs during the 52-week trial period, compared to 17% of placebo patients 1.
What were the results of the Adakveo STAND trial?
The global phase 3 STAND study, which again compared Adakveo against placebo, failed to demonstrate a statistically significant difference in the rate of VOCs between patient groups. Patients treated with Adakveo had on average 2.5 painful crises within a year of treatment - not meaningfully different from the 2.3 crises on average reported in the placebo group 3.
The unconvincing results of the STAND trial became the basis for Adakveo's revocation in the EU.
-
Oxbryta clinical trial results
In the HOPE trial, Oxbryta was tested against placebo. Efficacy was based on hemoglobin (Hb) response rates defined as a Hb increase of >1 g/dL from baseline to Week 24.
Oxbryta patients had a response rate of 51.1% vs 6.5% for the placebo patients. Indirect bilirubin decreased by 29.1% in Oxbryta patients, compared to a decrease by 2.8% in placebo patients. The percent reticulocyte count decreased by 18% in Oxbryta patients and increased by 6.8& in placebo patients 2.
As the shared data show, it's not possible to make a direct comparison between the two medicines, as different efficacy criteria were used in their clinical trials. Since both treatments approach sickle cell disease in a different way, it's likely that Oxbryta will not be a direct replacement of Adakveo, but rather a part of a new treatment plan that your doctor will make for you.

Adakveo vs Oxbryta: Safety and side effects
According to their prescribing information, these are the most common side effects of Adakveo and Oxbryta:
Adakveo side effects
- Nausea
- Joint pain
- Back pain
- Stomach pain
- Fever1.
During the SUSTAIN trial, 3% of patients experienced infusion-related reactions, characterized by symptoms such as headache, chills, vomiting, diarrhea, shortness of breath, or wheezing 1.
Oxbryta side effects
- Headache
- Diarrhea
- Stomach pain
- Nausea
- Rash
- Fever 2.
Fewer than 1% of clinical trial patients experienced serious hypersensitivity reactions including rash, mild shortness of breath, mild facial swelling, and eosinophilia (increase in the number of white blood cells)2.
Adakveo vs Oxbryta: Price comparison
When it comes to prices for medicines that are not yet approved or available, you should take these as indicative only. The final price may vary depending on your location, or the supplier.
Adakveo costs per year
The recommended dosage for Adakveo is 5 mg/kg of body mass every 4 weeks. This means 13 infusions per year. For a person who weighs 60 kg, this would require 13 infusions x 300mg of Adakveo, or 39 vials of the medicine.
At a price of around EUR 5,214 per 100 mg vial, the annual cost of an Adakveo treatment adds up to around EUR 203,346 5.
Oxbryta costs per year
The recommended dosage of Oxbryta for patients with body weight over 40 kg is 1,500 mg of Oxbryta once daily, equal to 3 tablets x 500 mg each.
A pack of 90 x 500 mg tablets costs around EUR 40,365, and is sufficient for one month, based on the recommended dosage above. Therefore, the annual cost of Oxbryta treatment adds up to EUR 484,380 6.
Where are Adakveo and Oxbryta approved?
Adakveo (crizanlizumab) is currently approved in several countries, including the USA, Canada and Australia 7,9,10. Its conditional marketing authorization in the EU was revoked in May, 2023 3.
Oxbryta (voxelotor) is approved in the USA, EU and Canada 4,8,9. However, within the EU, Oxbryta is not yet widely available on the market, as time between authorization and market launch can vary significantly per country.
Is Adakveo or Oxbryta not (anymore) approved or available in your country? If you and your doctor are of the opinion that these treatments might benefit you, get in touch with our team of Medicine Access experts. We can give you a personalized price quote for sourcing the medicine for you.
References:
- HIGHLIGHTS OF PRESCRIBING INFORMATION. Novartis, Accessed 27 September 2023.
- HIGHLIGHTS OF PRESCRIBING INFORMATION. Oxbryta, Accessed 27 September 2023.
- Adakveo | European Medicines Agency. European Medicines Agency, Accessed 27 September 2023.
- Oxbryta | European Medicines Agency. European Medicines Agency, Accessed 27 September 2023.
- Buy Adakveo (crizanlizumab) Online. Everyone.org, Accessed 27 September 2023.
- Buy Oxbryta (voxelotor) Online. Everyone.org, Accessed 27 September 2023.
- Adakveo. Therapeutic Goods Administration (TGA), Accessed 27 September 2023.
- FDA approves drug to treat sickle cell disease in pediatric patients. FDA, 17 December 2021.
- New Medicines Approved in 2019 - Meds Entry Watch. Canada.ca, 10 February 2021.
- FDA approves first targeted therapy to treat patients with painful complication of sickle cell disease. FDA, 15 November 2019.




