Enhertu for lung cancer: Where is it approved and how to get it?
Last updated: 15 July 2024
You can legally access new medicines, even if they are not approved in your country.
Learn howEnhertu (fam-trastuzumab deruxtecan-nxki) has been one of the cancer treatments to watch, ever since its first approval by the FDA in 2019 1.
As the safety and efficacy of Enhertu in ovarian cancer and colorectal cancer are still being investigated, the medicine is already approved for several indications. Including HER2-positive breast cancer, HER2-positive gastric cancer, and HER2-low breast cancer. The FDA also approved Enhertu for HER2-mutant non-small cell lung cancer. The CHMP in Europe has recently given a positive opinion for doing the same within the EU 2.
If you're a lung cancer patient in the UK or elsewhere outside of the USA and EU, you're probably wondering when Enhertu will also be available to you.
Here's everything to know about Enhertu for lung cancer, and what patients in the UK and rest of the world can expect.
What is the indication for Enhertu for lung cancer?
As a targeted therapy, Enhertu is only intended to treat cancers with a HER2 mutation. As such, the treatment applies to about 2% of all NSCLC patients 3.
Enhertu is indicated as monotherapy in adult patients with advanced NSCLC whose tumours have an activating HER2 (ERBB2) mutation. Enhertu is aimed at patients who have received a prior systemic therapy, and whose cancer has either spread to other parts of the body or cannot be removed surgically 2.
What is the success rate of Enhertu for lung cancer?
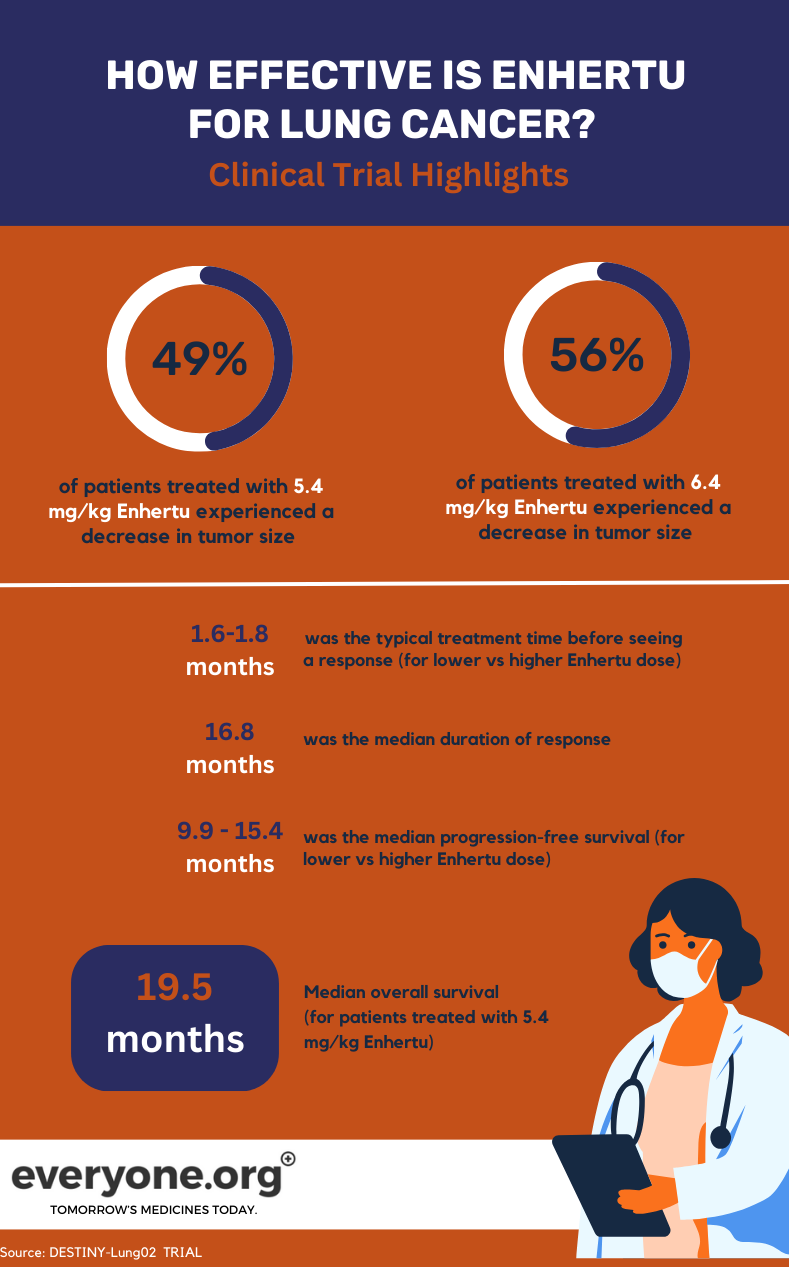
The ongoing DESTINY-Lung02 Phase II trial is investigating Enhertu's safety and efficiency in treating HER2-mutated NSCLC. In the trial, two doses of Enhertu (5.4 mg/kg and 6.4 mg/kg) are being compared.
For lung cancer patients, the key preliminary results from the DESTINY-Lung02 trial are:
- Tumours shrank for 49% of patients treated with the lower Enhertu dose and 56% of those treated with the higher dose.
- Tumours completely disappeared for 1% and 4% of the patients in the lower and higher dose group, respectively.
- The median duration of response was 16.8 months for patients in the lower Enhertu dose group. For patients in the other group, the DoR was reported as not estimable.
- The median time needed to observe initial treatment response was 1.8 months and 1.6 months in the lower and higher dose group, respectively.
- The median progression-free survival was 9.9 months at 5.4 mg/kg, and 15.4 months at 6.4 mg/kg 4.
What was the median survival for Enhertu patients?
For patients receiving the lower Enhertu dose in the clinical trial, median overall survival (OS) was 19.5 months. The OS was reported as not estimable for the higher dose group 4.
What are the side effects of Enhertu on the lungs?
Some severe, life-threatening or fatal cases of interstitial lung disease (ILD) have been reported in Enhertu patients. Patients with moderate renal impairment may be at a higher risk 6.
Within the DESTINY-Lung02 trial, ILD was reported in 12.9% of patients in the lower dose group, and in 28% of patients in the higher dose group. The majority of these cases were not severe. ILD of Grade 3 or higher was reported in 2% of patients in both patient groups.
Enhertu's safety profile for lung cancer is similar to its performance in other indications. Based on the lower incidence of adverse effects (38.6% vs 58%), the 5.4 mg/kg dose is considered the optimal treatment for NSCLC patients 4.

Where is Enhertu approved for lung cancer?
Currently, Enhertu is approved in the USA, EU, Israel, and Japan for the treatment of adult patients with unresectable or metastatic NSCLC whose tumours have activating HER2 (ERBB2) mutations 4.
Is Enhertu approved for lung cancer in the UK?
As of July 2024, not yet. A final draft guidance published by NICE in March 2024 announced that Enhertu will not be on the NHS until a cost-effective price is available. In the meantime, unfortunately, Enhertu is not available in the UK for lung cancer 7.
Can my doctor prescribe Enhertu for lung cancer if it's not approved yet?
The short answer is yes.
Enhertu has already been approved in multiple countries for the treatment of HER2-mutated NSCLC. Based on this, your doctor has the authority to prescribe the medicine for this indication even if it's not yet approved in your country.
When a doctor prescribes a medicine for treating a disease it's not approved for (yet), that's called off-label use. An off-label use prescription may be difficult to fill in some countries, depending on local regulations and availability. However, it's always possible to fill it using the Named Patient Import regulation.
Has your doctor made the decision to prescribe Enhertu for the treatment of your lung cancer? Our team at www.everyone.org can help you access the medicine. We specialise in sourcing and delivery of prescribed medicines unapproved or unavailable in a patient's country. Contact us, so we can help you.
References:
- Stewart, Judith. Enhertu (fam-trastuzumab deruxtecan-nxki) FDA Approval History. Drugs.com, 15 August 2022.
- Enhertu. European Medicines Agency, Accessed 06 November 2023.
- HER2 and Lung Cancer. American Lung Association, Accessed 6 November 2023.
- Enhertu demonstrated strong and durable tumour responses in previously treated HER2-mutant advanced lung cancer in DESTINY-Lung02 Phase II trial. AstraZeneca, 11 September 2023.
- Project information | Trastuzumab deruxtecan for treating HER2-mutated unresectable or metastatic non-squamous non-small-cell lung cancer after 1 or more therapies [ID3934] | Guidance. NICE, Accessed 6 November 2023.
- USPI-DB-04 CDX Update FINAL. Daiichi Sankyo, Accessed 6 November 2023.
- UK NICE rejects Daiichi Sankyo’s Enhertu for breast cancer, Pharmaceutical Technology, Accessed 15 July 2024.