Is dostarlimab available in the UK (and what to do in the meantime)?
Last updated: 05 February 2024

You can legally access new medicines, even if they are not approved in your country.
Learn howDostarlimab (commercially sold as Jemperli) is a novel anti-PD-1 therapy. It received conditional marketing authorization from the EMA and the MHRA in 2021 for the treatment of advanced or recurrent endometrial cancer [1, 6]. With that, it became the first anti-PD-1 therapy approved in Europe for this indication.
For patients with mismatch repair deficient (dMMR) tumors, this is a big milestone. Especially since innovation in the field has been lagging behind, according to Jack Harris, Vice-President UK Oncology at GSK [2].
However, those eager to start treatment with dostarlimab in the UK may need a bit more patience. It can still take some time before the medicine is widely available on the market.
Is dostarlimab approved in the UK?
Currently, dostarlimab is approved but not widely available on the UK market yet.
Dostarlimab is only available in the UK under the Early Access to Medicines Scheme (EAMS) [4]. Availability via EAMS is not equivalent to a full MHRA approval. The scheme aims to provide early access to new medications for severe illnesses or conditions that do not have effective treatment options available.
Good to know is that dostarlimab is available via EAMS only for the treatment of dMMR/MSI-H primary advanced or recurrent endometrial cancer. Its other FDA-approved indication, the treatment of dMMR/MSI-H advanced or recurrent solid tumors, is not yet recognised in the UK.
Is dostarlimab available on the NHS?
In February 2022, the NHS announced that dostarlimab would be provided to an estimated 124 women with dMMR/MSI-H endometrial cancer each year.
The National Institute for Health and Care Excellence (NICE) supports dostarlimab's use within the Cancer Drugs Fund for patients who have previously had platinum-based chemotherapy.
Dostarlimab's inclusion in the Cancer Drugs Fund allows for the collection of more extensive data and long-term evidence. However, at this point, dostarlimab is not yet recommended for routine NHS use.
What if you are not one of the 124 women who receive early access to this novel immunotherapy each year? Or, if you require the medication for dMMR/MSI-H solid tumors rather than endometrial cancer? In these cases, dostarlimab is currently unavailable to you in the UK through the NHS.
When will dostarlimab be available in the UK?
Receiving the MHRA's conditional marketing authorization was only the first step to dostarlimab's availability on the UK market. Local pricing decisions, as well as full NHS coverage need to also be considered and decided [5].
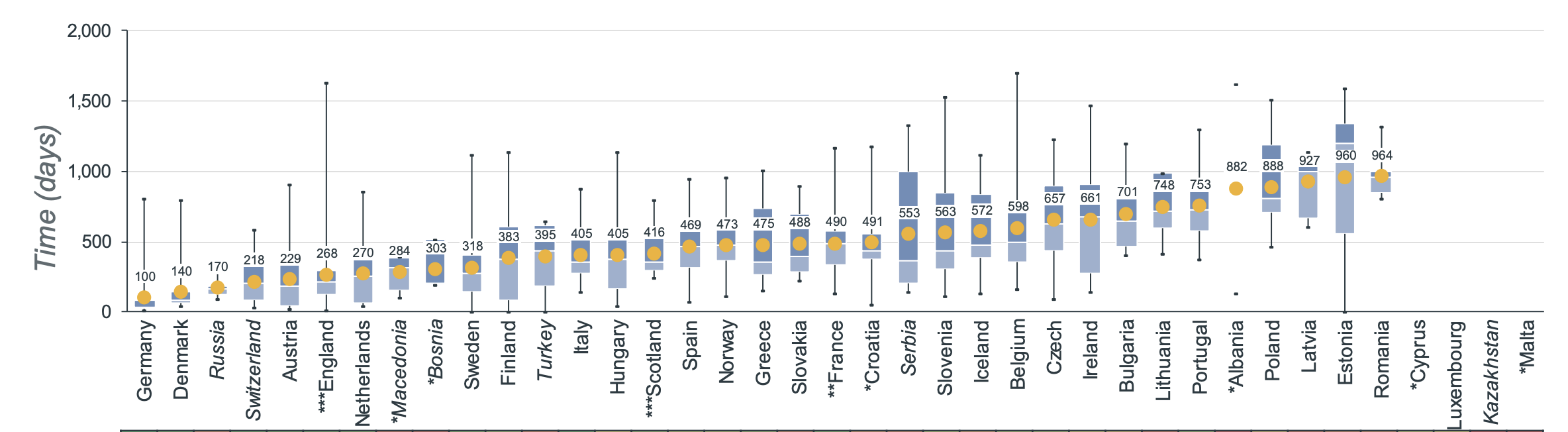
The average time needed from the moment a medicine gets EMA approval to the moment it's available on the market varies a lot per country. The European average for oncology treatments in 2022 was 511 days between EMA marketing authorization and date of wide availability. In England, the average time to availability is 268 days, and in Ireland - 661 days [3].
In this context, it might still take some time before dostarlimab is widely available on the UK market.

How can you get dostarlimab in the UK?
Are you a patient in the UK with endometrial cancer or advanced dMMR/MSI-H solid tumor? If your doctor recommends treatment with dostarlimab, and you don't qualify for early access to the treatment, you still have options.
When a medicine is unapproved in a patient's country, or it's approved but not yet available (as in dostarlimab's case in the UK), you can access it via the Named Patient Import regulation.
Everyone.org specializes in helping people access the latest medicines via this regulation. If you have a prescription from your treating doctor for Jemperli (dostarlimab), you're impatient to start your treatment plan, and would like us to help you access the medicine immediately, contact us.
References:
- Jemperli | European Medicines Agency. European Medicines Agency, 21 April 2021.
- Cooper, Emma. UK patients granted early access to GSK’s endometrial cancer treatment. Pf Media, 10 July 2023.
- EFPIA Patients W.A.I.T. Indicator 2021 Survey. EFPIA, Accessed 25 September 2023.
- Early Access to Medicines Scientific Opinion - Public Assessment Report Product Dostarlimab EAMS indication Dostarlimab. GOV.UK, 29 June 2023.
- Ewbank, Leo. Access to new medicines in the English NHS. The King's Fund, 28 October 2020.
- Parsons, Lucy. GSK's PD-1 inhibitor Jemperli approved in the UK. Pharma Times, 7 June 2021.