Can Enhertu Treat Ovarian Cancer? All the Data, None of the Hype.
Last updated: 15 July 2024
You can legally access new medicines, even if they are not approved in your country.
Learn howEnhertu (fam-trastuzumab deruxtecan-nxki) was first approved by the FDA in 2019 1. At that point, it was indicated for the treatment of HER2-positive breast cancer. Since then, Enhertu has been approved for more indications. These include HER2-positive gastric cancer, HER2-low breast cancer, and HER2-mutant non-small cell lung cancer. Since April 2024, the treatment is also FDA-approved for use across all HER2-positive tumours5.
In this context, Enhertu has gathered a lot of media attention. As a natural consequence of the hype, cancer patients everywhere are wondering whether Enhertu can also be applicable to them.
As always, we're here to cut through the noise and look at the available data. In this article, we'll review all there is to know about Enhertu for ovarian cancer.
What type of cancer does Enhertu treat?
Patients everywhere have been wondering if Enhertu can cure all cancer. Unfortunately, that's not the case. And it's not the case by design. Enhertu is a targeted therapy specifically for:
- Tumors with a HER-2 expression. HER-2 is a protein found on the surface of different types of cells in the body. It helps with normal cell growth. However, in certain cancers, it can become overactive due to gene changes or mutations. HER2 overexpression affects between 2% and 66% of ovarian cancer cases 5.
- Tumors that have spread to other parts of the body and/or are inoperable 4. Future clinical trials may involve Enhertu at earlier stages of cancer treatment 6. However, this is currently not the case.
How effective is Enhertu for ovarian cancer?
The ongoing phase 2 DESTINY-PanTumor02 trial is focused on testing Enhertu's activity in various HER2-positive solid cancers. One of them is ovarian cancer. The interim results of the trial were shared in June 2023, and are showing some promise.
For ovarian cancer patients, the key preliminary outcomes of the trial are:
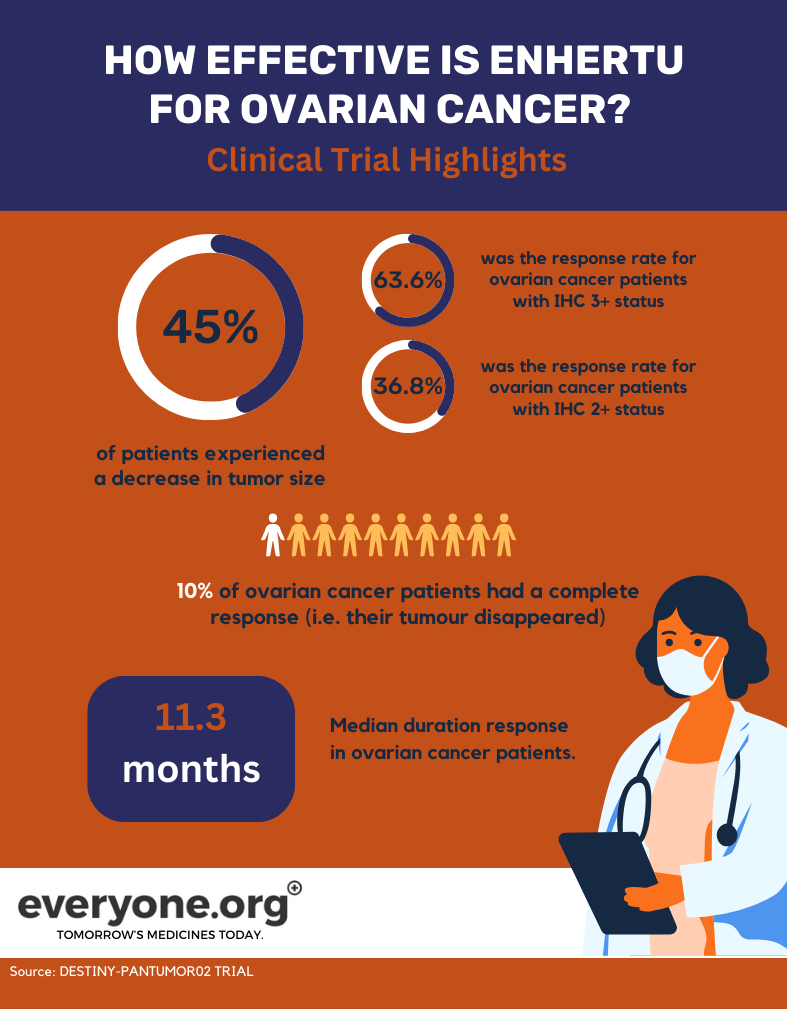
- Overall response rate was 45.0%. Meaning that 45% of patients treated with Enhertu had their tumours shrink. For patients with a HER2 status of IHC 3+, the overall response rate was 63.6%. For those with IHC 2+ status, the response rate was 36.8% 3.
- 10% of ovarian cancer patients had a complete response. In other words, their tumour completely disappeared 3.
- After 12 months, 45.8% of ovarian cancer patients with tumors that decreased in size or disappeared still reported experiencing this benefit3.
- The median duration of response in ovarian cancer patients was 11.3 months3.
Overall, these results can be considered encouraging. The long duration of responses is a particularly notable result, according to Dr. Meric-Bernstam (Chair of the Department of Investigational Cancer Therapeutics at the USA MD Anderson Cancer Center) 3.
Based on these clinical study results, the FDA in the USA granted Enhertu a its first-ever approval of a medicine to be used across all cancers with a HER2 expression.

When will Enhertu be approved for ovarian cancer?
As of April 2024, Enhertu is approved for all HER2-expressing cancers, including ovarian cancer.
The DESTINY-PanTumor02 trial is ongoing, with final results expected in April 2027 7. However, the preliminary results of the study were already sufficient for the FDA's recent pan-tumor approval.
If you're based outside the USA, Enhertu's approval status for ovarian cancer may be different.
Can my doctor prescribe Enhertu for ovarian cancer?
The best answer currently is, maybe.
As of July 2024, Enhertu has been approved only in the USA for the treatment of HER2-positive ovarian cancer. However, even in other countries, your doctor has the authority to prescribe the medicine for this indication anyway. They could do this based on the preliminary results of the DESTINY-PanTumor02 clinical trial, and on the specifics of your case.
When a doctor prescribes a medicine for treating a disease it's not locally approved for (yet), that's called off-label use. An off-label use prescription may be difficult to fill in some countries, depending on local regulations and availability. However, it's always possible to fill it using the Named Patient Import regulation.
Has your doctor made the decision to prescribe Enhertu for the treatment of your ovarian cancer? Our team at www.everyone.org can help you access the medicine. We specialize in sourcing and delivery of prescribed medicines unapproved or unavailable in a patient's country. Contact us, so we can help you.
References:
- Stewart, Judith. Enhertu (fam-trastuzumab deruxtecan-nxki) FDA Approval History. Drugs.com, 15 August 2022.
- Breakthrough Therapy. FDA, 4 January 2018.
- Doherty, Kyle. Enhertu Is Promising for Difficult-to-Treat Solid Cancers. Cure Today, 6 June 2023.
- USPI-DB-04 CDX Update FINAL. Daiichi Sankyo, Accessed 3 October 2023.
- Teplinsky, Eleonora, and Franco Muggia. EGFR and HER2: is there a role in ovarian cancer?, Translational Cancer Research, 13 February 2015.
- Liu, Angus. ASCO: Enhertu delivers 'very compelling' pan-tumor activity. Fierce Pharma, 5 June 2023.
- A Phase 2 Study of T-DXd in Patients With Selected HER2 Expressing Tumors (DPT02), ClinicalTrials.gov, Accessed 03 October 2023.