Is dostarlimab available in Belgium (and what to do in the meantime)?
Last updated: 15 January 2024

You can legally access new medicines, even if they are not approved in your country.
Learn howDostarlimab (commercially sold as Jemperli) is a novel anti-PD-1 therapy. It received conditional marketing authorization from the EMA in 2021 for the treatment of advanced or recurrent endometrial cancer [1]. With that, it became the first anti-PD-1 therapy approved in Europe for this indication.
For patients with mismatch repair deficient (dMMR) tumors, this is a big milestone. Especially since innovation in the field has been lagging behind, according to Jack Harris, Vice-President UK Oncology at GSK [2].
However, those eager to start treatment with dostarlimab in Belgium may need a bit more patience. It can still take some time before the medicine is widely available on the market.
Is dostarlimab available in Belgium?
Currently, dostarlimab isn't widely available on the Belgian market.
After its conditional marketing authorization in 2021, dostarlimab has been available in Belgium as part of two Compassionate use programs:
- One focused on dostarlimab as a single agent in the treatment of recurrent or advanced dMMR/MSI-H endometrial cancer which has progressed after prior treatment. This program was closed in 2022 [3].
- One focused on dostarlimab in combination with chemotherapy as first-line treatment of primary advanced or recurrent dMMR/MSI-H endometrial cancer. This program is currently still active [3].
Dostarlimab also has another FDA-approved indication, for use in treating dMMR/MSI-H advanced or recurrent solid tumors. For this indication, it is not currently approved by the EMA, and is not available in Belgium.
When will dostarlimab be available in Belgium?
Receiving the EMA's full marketing authorization (expected in 2024) will be the first step to dostarlimab's availability on the Belgian market. If it’s approved by the FAMHP for inclusion in the care pathway, decisions on local pricing and on health insurance coverage will also need to take place.
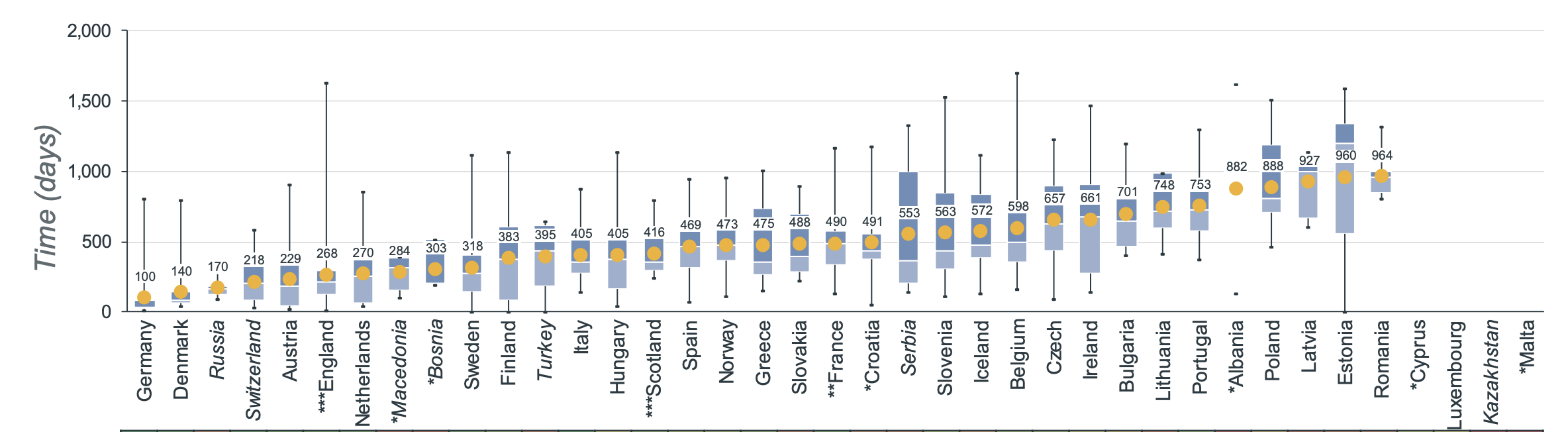
The average time needed from the moment a medicine gets EMA approval to the moment it's available on the market varies a lot per country. The European average for oncology treatments in 2022 was 511 days between EMA marketing authorization and date of wide availability. In Belgium, the average time to availability is 598 days [4].
In this context, it might still take some time before dostarlimab is widely available on the Belgian market.

What can you do until dostarlimab comes to Belgium?
Are you a patient in Belgium with endometrial cancer, who doesn’t qualify for the Compassionate use program? Or are you a patient with an advanced dMMR/MSI-H solid tumor? If your doctor recommends treatment with dostarlimab, you have options.
When a medicine is unapproved in a patient's country, or it's approved but not yet available, you can access it via the Named Patient Import regulation.
Everyone.org specializes in helping people access the latest medicines via this regulation. If you have a prescription from your treating doctor for Jemperli (dostarlimab), you're impatient to start your treatment plan, and would like us to help you access the medicine immediately, contact us.
References:
- Jemperli | European Medicines Agency. European Medicines Agency, 21 April 2021.
- Cooper, Emma. UK patients granted early access to GSK’s endometrial cancer treatment. Pf Media, 10 July 2023.
- Compassionate use - Medical need. FAMHP, Accessed 25 September 2023.
- EFPIA Patients W.A.I.T. Indicator 2021 Survey. EFPIA, Accessed 25 September 2023.